Infective Endocarditis and Prevention
Michael Gewitz
Kathryn A. Taubert
Infective endocarditis (IE) is a disease that is associated with considerable morbidity and mortality and remains a dreaded complication of structural heart disease. Although relatively rare in children, this cardiac infection with protean manifestations continues to have a disproportionate influence on clinical practice. Reported mortality rates are much lower now than in the preantibiotic era; however, overall morbidity and the expense burden of prolonged and often intense medical and surgical therapies remain formidable.
Major advances in understanding this disease process have been made over the years. Additionally, the development and refinement of echocardiographic techniques have contributed to better diagnosis and management of endocarditis. Increasingly precise clinical criteria for the diagnosis of IE have been established (1,2) that assist physicians in making a better objective assessment of the varied clinical manifestations of this disease process.
Definition
IE is a microbial infection of the endocardial (endothelial) surface of the heart. Native or prosthetic heart valves are the most frequently involved sites. Endocarditis also can involve septal defects, the mural endocardium, or intravascular foreign devices such as intracardiac patches, surgically constructed shunts, and intravenous catheters. Infective endarteritis is a similar clinical illness involving arteries, including the ductus arteriosus, the great vessels, aneurysms, and arteriovenous shunts.
At one time, endocarditis was classified as acute or subacute, but the recent tendency has been to avoid this terminology. It is preferable to describe the disease based on the etiologic agent involved. Low-virulence organisms such as α-hemolytic streptococci, enterococci, or coagulase-negative staphylococci usually cause a prolonged subacute form of the illness. On the other hand, Staphylococcus aureus and other pyogenic bacteria, such as Streptococcus pneumoniae or b-hemolytic streptococci, are usually associated with a more virulent or acute clinical illness.
Epidemiology
The epidemiology of heart disease in children has changed during the past several decades as has the epidemiology of IE itself. While it is difficult to get exact incidence data for IE because it is not a notifiable disease, the incidence is believed to have increased since the mid-1900s. The reported US incidence from the early 1980s was approximately 1 in 1,280 (3). In a review of several published studies between 1986 and 1995, the estimated incidence in children overall was 0.3 per 100,000 children per year with a mortality of 11.6% (4). In recent years, a multicenter study including children under 18 years of age hospitalized from 2003 to 2010 with IE found the annual US rate to be between 0.05 and 0.12 cases per 1,000 pediatric hospital admissions (5).
The current incidence of IE in children reflects increased survival of patients with cardiovascular disease, paralleling advances in surgical and medical approaches to congenital heart disease (CHD) during the past several decades as well as aggressive treatment regimens in neonatal and pediatric intensive care units. Whereas rheumatic heart disease was a major underlying cardiac condition in US children with endocarditis until the 1970s, it has become an unusual underlying cardiac condition in more recent years. This is not true in developing countries, however, where endocarditis remains an important complicating factor in individuals with rheumatic heart disease.
In general, CHD conditions, such as ventricular septal defect, patent ductus arteriosus, aortic valve abnormalities, and tetralogy of Fallot, are now the more common underlying conditions. An increasing proportion of children with IE have had previous corrective or palliative surgery for CHD, with or without implanted vascular grafts, patches, or prosthetic cardiac valves (6), and various reports have indicated that 50% to 70% of children with CHD and IE have had previous cardiac surgery (3,7,8,9,10).
In neonatal IE, in contrast, most cases occur in structurally normal hearts. Although relatively uncommon, increasing numbers of cases of neonatal IE have been reported since the 1980s. This reflects marked increases in cardiovascular interventions (surgical and nonsurgical) in newborns and young infants with concomitant increased use of prosthetic intravascular devices and insertion of long-term indwelling central venous catheters (6).
In summary, patients with underlying cardiovascular disease may develop endocarditis at any age—in childhood, adolescence, or adulthood. In a pooled series of 723 children with congenital heart defects who developed endocarditis, the defects most frequently associated with IE were ventricular septal defect, tetralogy of Fallot, and aortic stenosis (7). Two important reviews have characterized these epidemiologic changes succinctly. Yoshinaga et al. (11) identified increasing morbidity and mortality from IE in infants with CHD less than 1 year of age. Rosenthal et al. (12) also found that it is younger children with CHD, especially those who have undergone surgical treatment, who constitute increasing number of IE patients as compared to earlier healthcare eras.
Microorganisms
Most cases of endocarditis are caused by a relatively small number of microorganisms (Table 62.1). Experimental data and clinical observations indicate that some bacteria are more commonly associated with endocarditis than others. One of the most logical and intriguing explanations relates to bacterial adherence. Following careful in vitro studies, Gould et al. (30) found that bacteria most frequently responsible for endocarditis (e.g., viridans streptococci) display a propensity for adherence to canine or human valves. In contrast, gram-negative organisms, seldom responsible for endocarditis, adhere rather poorly in this in vitro system.
Gram-positive cocci account for about 90% of recoverable bacteria in adult patients. Viridans group streptococci are still responsible for most cases of endocarditis in all age groups in developing countries where, as a predisposing condition, rheumatic heart disease is prevalent. Staphylococci (S. aureus and coagulase-negative staphylococci) account for more cases than do viridans group streptococci in developed countries where health care–associated infections are becoming more prevalent (13).
TABLE 62.1 Etiologic Agents of Infective Endocarditis in Infants and Children | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||
Similar trends have been noted in the pediatric age group (6,7,10,14). One study examined the prevalence of IE among children with S. aureus bacteremia. The prevalence in definite IE was approximately 12%, and in definite or possible IE was 20%. Most of these children developed bacteremia from an infected intravascular device (15). Overall, enterococcal endocarditis occurs much less frequently in children than in adults.
Gram-negative organisms cause <10% of the endocarditis in children. However, neonates, immunocompromised patients, and injection drug users (IDUs) are at an increased risk for gram-negative bacterial endocarditis. Among the fastidious gram-negative bacilli of the HACEK group (Haemophilus parainfluenzae, H. aphrophilus, H. paraphrophilus, H. influenzae, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae, and K. denitrificans), Haemophilus species are more common than the others in children, frequently affect previously damaged valves, and cause a subacute course of endocarditis. Rarely, Neisseria gonorrhoeae can cause endocarditis, presenting as an acute illness and affecting previously normal valves. Commonly, valvular destruction results from this organism. In IDUs, other organisms isolated include S. aureus and occasionally Candida or other fungal species.
In instances of prosthetic valve endocarditis, the infective organisms differ depending on whether endocarditis occurs early (<2 to 3 months after surgical procedure) or late. Prosthetic valve endocarditis frequently is caused by S. aureus or coagulase-negative staphylococci. These infections often are implanted at the time of surgery and are seen ∼60 days after cardiac surgery, but coagulase-negative staphylococci may be present as late as 1 year after surgery.
Anaerobic organisms rarely cause endocarditis in children. Some individuals with the clinical picture of endocarditis, but with sterile routine blood cultures, may be infected with anaerobic organisms, which are not readily recovered by the usual culture methods in the clinical laboratory.
Fungal endocarditis is relatively unusual in children although it is one of the most feared forms of endocarditis. Complications, especially embolization, are frequent. Candida species are the most common organisms recovered; Aspergillus species, Torulopsis glabrata, and some other fungi (Histoplasma, Coccidioides, Cryptococcus) also have been reported. Fungal endocarditis is often noted in narcotic addicts or after cardiac surgery, but it also occurs in immunocompromised individuals and in neonates. In neonates, this infection may be a complication of modern intensive care measures, including hyperalimentation fluid infusion, use of broad-spectrum antibiotics for a prolonged time, and extended use of indwelling venous catheters. The mortality rate from fungal endocarditis is high, even with intensive medical and surgical therapy (6,14).
Approximately 5% to 10% of patients with endocarditis have negative blood cultures. Culture-negative endocarditis (CNE) is diagnosed when a patient has clinical and/or echocardiographic evidence of IE but blood cultures are repeatedly negative. The most common causes of culture-negative IE are current or recent antibiotic therapy or infection caused by a fastidious organism that grows poorly in vitro (6). Many of these individuals demonstrate subsequent proof of endocarditis, either in the operating room or at necropsy. Consultation with the clinical microbiologist is invaluable in looking for unusual and fastidious organisms as molecular methods may be required in addition to standard blood cultures (16). The clinician should carefully evaluate such cases for the possibility of other diseases. For example, IE can be confused with other causes of postoperative fever (e.g., postpericardiotomy syndrome) and may be appropriately included in the differential diagnoses of collagen vascular diseases or even certain oncologic diseases presenting in childhood.
Pathogenesis
Several independent factors and events are requisites for the development of IE. A pre-existing congenital or acquired lesion of the heart or great vessels is usually present, although in some instances, such as in IDUs and in patients with intravenous catheters, endocarditis may develop in the absence of cardiovascular structural abnormalities. The sequence of events resulting in endocarditis includes damage to the endothelium, formation of nonbacterial thrombotic endocarditis (NBTE) on the surface of the damaged endothelium, occurrence of a transient bacteremia, adherence of these bacteria to the NBTE, and subsequent proliferation of the bacteria within a vegetation.
Careful clinical and pathologic studies of endocarditis have defined the underlying structural cardiac or great vessel abnormalities that are the most frequent sites of infection. The risk of endocarditis varies for different underlying conditions (Table 62.2). A review of available reports by Steckelberg and Wilson (17) suggests that the incidence of endocarditis in the general population is approximately five cases per 100,000 person-years. In high-risk groups, the incidence is substantially higher (300 to 2,160 cases per 100,000 person-years). The incidence in the moderate risk groups ranges from 50 to 440 cases per 100,000 person-years.
Virtually all vegetations occur in areas where there is a pressure gradient with resulting turbulence of blood flow. Turbulent blood flow produced by certain types of congenital or acquired heart disease, such as flow from a high- to low-pressure chamber or across a narrowed orifice (e.g., where a jet through a small ventricular septal defect hits the right ventricular wall) traumatizes the endothelium. The sites of high-velocity jets where most IE vegetations occur are most often on the atrial side of the atrioventricular valves and the
ventricular side of the semilunar valves. Intact cardiac endothelium is a poor stimulator of blood coagulation and is weakly receptive to bacterial attachment, whereas damaged or denuded endothelium is a potent inducer of thrombogenesis. Thrombogenesis at such a site results in the deposition of sterile clumps of platelets, fibrin, and occasionally red blood cells and the formation of NBTE. This provides an environment to which bacteria can adhere and eventually form an infected vegetation. NBTE also can be produced in children with indwelling intravenous catheters positioned in the right side of the heart. Such catheters may traumatize the endocardium or valvular endothelium, exposing the subendothelial collagen. Much has been learned about the pathogenesis of the developing site of infection by study in experimental animals of NBTE. Use of a polyethylene catheter in a rabbit model, for example, yielded important information. Very shortly after the vascular endothelium is injured by the catheter, platelets and fibrin will adhere to the site of injury. This meshwork continues to grow with further accumulation of platelets and fibrin; very few leukocytes are involved. Following the initial deposition of platelets and fibrin, thrombus formation occurs. Certain bacteria such as staphylococci and streptococci, commonly implicated in endocarditis, are potent stimuli of platelet aggregation. In addition, the lysosomal granules of platelets may release hydrolytic enzymes or other active proteins that may potentiate the process.
ventricular side of the semilunar valves. Intact cardiac endothelium is a poor stimulator of blood coagulation and is weakly receptive to bacterial attachment, whereas damaged or denuded endothelium is a potent inducer of thrombogenesis. Thrombogenesis at such a site results in the deposition of sterile clumps of platelets, fibrin, and occasionally red blood cells and the formation of NBTE. This provides an environment to which bacteria can adhere and eventually form an infected vegetation. NBTE also can be produced in children with indwelling intravenous catheters positioned in the right side of the heart. Such catheters may traumatize the endocardium or valvular endothelium, exposing the subendothelial collagen. Much has been learned about the pathogenesis of the developing site of infection by study in experimental animals of NBTE. Use of a polyethylene catheter in a rabbit model, for example, yielded important information. Very shortly after the vascular endothelium is injured by the catheter, platelets and fibrin will adhere to the site of injury. This meshwork continues to grow with further accumulation of platelets and fibrin; very few leukocytes are involved. Following the initial deposition of platelets and fibrin, thrombus formation occurs. Certain bacteria such as staphylococci and streptococci, commonly implicated in endocarditis, are potent stimuli of platelet aggregation. In addition, the lysosomal granules of platelets may release hydrolytic enzymes or other active proteins that may potentiate the process.
TABLE 62.2 Relative Risk of Endocarditis for Various Cardiovascular and Underlying Conditions | ||
|---|---|---|
|
Experimental studies have shown that circulating microorganisms are entrapped within this meshwork, becoming the nidus of infection. This usually occurs distal to the pressure gradient. The exception to this is valvular aortic stenosis, where the site of the vegetation is usually on the ventricular side of the aortic valve. A possible explanation for this finding is that in almost all instances of aortic stenosis there is at least some degree of aortic insufficiency. The ability of various microorganisms to adhere to specific sites determines the location of the infection. Mediators of bacterial adherence serve as virulence factors in the pathogenesis of IE. Numerous bacterial surface components present in streptococci, staphylococci, and enterococci have been shown in animal models of endocarditis to function as critical adhesions. Bacteria adhering to the vegetation stimulate further deposition of fibrin and platelets on their surface. Within this secluded focus, the buried microorganisms multiply rapidly. For S. aureus these adhesions have been termed MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) (18).
The original site of infection subsequently changes in character. This change depends on several factors, including the microorganism involved. In endocarditis caused by a-hemolytic streptococci, the large colonies of bacteria become encased in an organizing mass of fibrin. The fibrin barrier has a direct effect on two important factors in the defense against infection: the prevention of the invasion by phagocytic leukocytes and the difficulty in penetration of the vegetation by antimicrobial agents. For reasons that are not fully appreciated, this type of vegetation formation does not frequently occur with some of the more virulent bacteria, such as S. aureus, where the infection rapidly destroys the valve or invades the myocardium with subsequent abscess formation.
There have been substantial gains within the past several years in the understanding of the pathogenesis of endocarditis largely because of the availability of newer molecular biologic techniques. These techniques have allowed the examination of individual virulence factors of gram-positive cocci and the investigation of important host–cell interactions with microorganisms. Several specific surface structures of staphylococci, streptococci, and enterococci have been identified as markers of virulence (19). Mucosal surfaces are populated by endogenous bacteria. Transient bacteremia can be induced by trauma to a mucosal surface during various dental, oral, and surgical procedures; however, spontaneous bacteremia may also occur (17). The bacteremia associated with various tissue manipulations, including dental and surgical procedures, has been carefully studied. Spontaneous bacteremia also has been noted to occur after tooth brushing, chewing hard foods, or other daily life events. Many dental procedures have been associated with bacteremia, particularly procedures known to induce gingival or mucosal bleeding. Transient bacteremia caused by viridans group streptococci and other oral microflora following tooth extraction may reach 80%. In experimental animals, large doses of bacteria are generally needed to induce endocarditis. In humans, it is likely that the dose in spontaneous transient bacteremia may be too small to lead to implantation of bacteria resulting in IE (20).
Following successful medical therapy, the cardiac lesions of endocarditis usually heal, although important residua can remain. Experimental studies in rabbits suggest that the resolution process includes endothelialization of the affected surface; phagocytosis of bacterial debris, sometimes with calcification; and subsequent organization by fibroblasts. Resulting hemodynamic abnormalities depend on the site of infection, the specific damage caused by the active vegetation, and the size and location of the abscess.
The immediate consequences of endocarditis, including vegetation formation, hemodynamic alterations, and the clinical syndrome, are only part of an evolving complex disease entity. Distal manifestations of the disease in the past were considered to be the results of embolic phenomena. It is now recognized that additional mechanisms are involved in the pathogenesis of endocarditis that lead to peripheral manifestations. Many important extracardiac findings in endocarditis are related to immunologic mechanisms. Rheumatoid factor is present for 6 weeks or longer in sera of about half of the patients with endocarditis. This is considered to be due to a gradual hyperimmunization of the host. Rheumatoid factor is more frequently found in patients with endocarditis related to a-hemolytic streptococci or coagulase-negative staphylococci (low-virulence organisms) than in those caused by S. aureus (more virulent organisms). There is also correlation of the duration of infection with the presence of this antiglobulin. Rheumatoid factor tends to disappear from the sera with successful therapy. Immunologic mechanisms also underlie other clinical manifestations of the disease including the skin, subcutaneous tissues, and eye findings noted below (Clinical Features).
Another immunologic consequence of endocarditis is the development of circulating immune complexes in the sera of patients. This is due to extended exposure to foreign antigen, and these immune complexes disappear after successful antimicrobial therapy. Although deposition of immune complexes in renal parenchyma can occur, their precise role in pathogenesis has not been fully defined. The nephritis seen in patients with endocarditis may manifest itself microscopically as either focal or diffuse glomerulonephritis. In the focal lesion, there is often segmental fibrinoid necrosis of isolated lobules of the glomerular tuft. In the more diffuse form, there is marked cellular proliferation with interstitial round cell infiltrates. Immunofluorescence studies show granular deposits in the glomerular basement membrane and mesangium, usually associated with complement and immunoglobulin G (IgG) deposits, although IgA, IgM, and fibrinogen also have been demonstrated. Urinalysis results may be normal, but hematuria, cylindruria, and pyuria have been reported. Compromise of renal function may occur and appears to be more common in adults than in children. In addition to immune mechanisms, the kidney is an extracardiac site frequently affected in patients with endocarditis because of microscopic and macroscopic emboli of pathologic lesions. While many emboli are reportedly sterile, abscess formation also has been reported following septic embolization to the kidney. Abscess formation in other vital organs occurs as well (see “clinical features” below) and is involved in the pathogenesis of many of the life-threatening sequelae of active IE.
Clinical Features
Most clinical manifestations and complications of endocarditis are directly related to hemodynamic and structural changes caused by the local infection, to embolization from vegetations, or to immunologic reactions by the host. Bacteremia itself can also cause clinical findings such as fever and systemic toxicity. Endocarditis simulates a wide variety of disorders, including other infectious diseases, malignancies, and connective tissue diseases. It should be part of the differential diagnosis of any unusual or febrile illness in patients with underlying heart disease. If the diagnosis is not made promptly, the disease may escape detection until the process is far advanced with irreparable damage.
The clinical features differ, in part, depending on the primary infection site. Findings also may be different in children compared with adults. Most cases of endocarditis in adults are valvar, but endocarditis in children with congenital heart lesions may often involve other structures such as mural endocardium, patent ductus arteriosus, arteries or other vascular sites such as conduits or surgical shunts.
Endocarditis involving the left side of the heart frequently results in peripheral embolization, leading to ischemia, infarction, or mycotic aneurysms. In these cases, specific clinical findings depend on the localization of the emboli. In children, embolization from the right heart may be no less frequent, but such emboli, if small, are not as easily appreciated clinically because of filtration by the lungs. However, large pulmonary emboli may complicate endocarditis of the tricuspid valve.
Table 62.3 lists clinical findings and usual laboratory findings seen in patients with endocarditis. Fever is the most common finding in all patients with endocarditis, with the exception of neonates, and can vary depending on the infecting organism. When hemolytic streptococci are the causative agents, for example, fever is often low grade, reaching a maximum of 39°C. In contrast, endocarditis caused by S. aureus is frequently associated with high, spiking temperature elevations of ≥40°C.
Children with “subacute” endocarditis usually display slowly progressive nonspecific symptoms, including myalgias, arthralgias, headache, and general malaise. Fever may be relapsing and low grade. There is often a marked diminution in appetite. In contrast, with the acute form of endocarditis a toxic course is the rule with high fever, systemic debilitation, and more overt hemodynamic changes on presentation.
TABLE 62.3 Clinical and Laboratory Findings in Patients with Endocarditis | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||
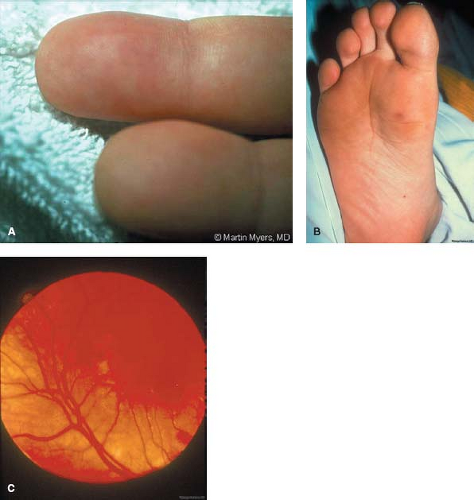
With diligent auscultation, new or changing murmurs are often heard. Since it may be difficult to recognize changes in a patient who has a pre-existing murmur, serial auscultation is essential. This will assist not only with diagnosis but also with management. For example, increased intensity of the diastolic murmur of aortic insufficiency should alert the examiner to the possibility of progressive aortic valvar deterioration, which can lead to worsening left ventricular dysfunction, heart failure, and to the possible need for early valve replacement. Similarly, diminution of a continuous murmur in a cyanotic child with a prosthetic systemic-to-pulmonary shunt may be a sign of endocarditic involvement of the graft. Extracardiac findings can include splenomegaly, which may be seen in most instances when the disease has been present for weeks or months. Neurologic findings are present in about 20% of children with endocarditis and may simulate the picture of an abscess, an infarct, or aseptic meningitis. Renal abnormalities, including proteinuria, hematuria, and leukocyturia, can occur in the presence of emboli or in association with endocarditis-related immune complex deposition, as noted previously. The classically described signs such as small, tender, raised lesions on the pads of the fingers and toes (Osler nodes, Fig. 62.1A); small, nontender, flat erythematous lesions on the palms or soles (Janeway lesions, Fig. 62.1B); retinal hemorrhages with a central white spot (Roth spots, Fig. 62.1C); and splinter hemorrhages can also occur but are relatively rare in children.
Neonates represent a unique group in that many may have relatively few specific symptoms. In those newborns that are symptomatic, systemic hypotension, clinical signs compatible with generalized sepsis, or focal neurologic findings from central nervous system embolization may be clues to the development of endocarditis. Neonates appear particularly prone to peripheral septic embolization and the development of satellite infections including meningitis and osteomyelitis.
Laboratory Diagnosis
The blood culture is the most valuable aid in making the diagnosis of endocarditis. Although a positive blood culture in a child with underlying or predisposing cardiac disease or a history of previous endocarditis does not necessarily indicate endocarditis, it is imperative that the diagnosis be considered in such a patient.
It is not possible to specify the exact number of cultures that should be obtained in each situation. The collection of three separate sets of blood cultures, each from a separate venipuncture over a 24-hour period, is adequate in most cases. In patients in whom the diagnosis of endocarditis is highly suspect, and the clinical situation is changing, arbitrary therapy should be considered after blood cultures have been obtained over an appropriate time period. In some situations, however, making careful observations and obtaining more blood cultures before initiating antibiotic therapy are appropriate.
Since the bacteremia in patients with IE is usually continuous, it is not necessary to obtain blood cultures only upon a temperature elevation. The volume of blood to be collected is another consideration, particularly in instances where there is a likely low magnitude of bacteremia. Usually 20 to 30 mL of blood are collected from an adult patient, but this is not possible in a small child. Thus, 1 to 3 mL in infants and young children and 5 to 7 mL in older children are adequate, depending on the blood culture detection system (6). It is rare for IE to be caused by anaerobic bacteria; therefore, the emphasis is usually on inoculating blood into bottles designed for aerobic incubation. Usually, three blood cultures are obtained by separate venipunctures on the first day, and if there is no growth by the second day of incubation, two more may be obtained. It is usually not necessary to obtain more than five blood cultures over 2 days unless the patient received prior antibiotic therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree