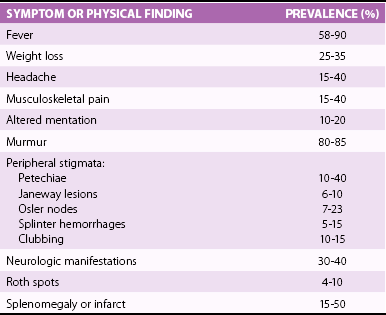
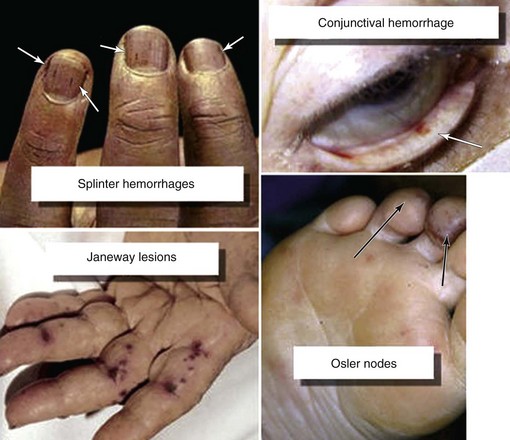
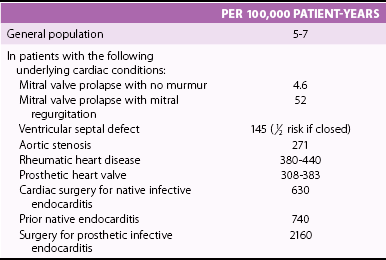
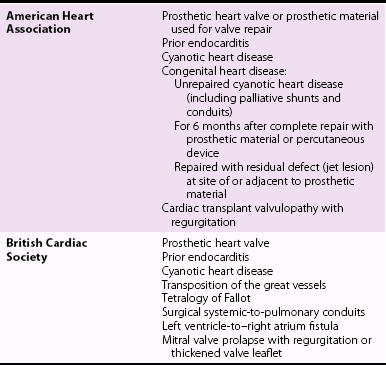
Chapter 25 Incidence of Infective Endocarditis and Associated Mortality Groups at High Risk for Development of Infective Endocarditis, and Determination of Prognosis PATHOPHYSIOLOGY AND PATHOGENESIS Evolution of the Duke Criteria Emerging Roles for Biomarkers and Polymerase Chain Reaction Analysis Transthoracic and Transesophageal Echocardiography Echocardiographic Features in Infective Endocarditis MORTALITY, MORBIDITY, AND ROLE OF SURGICAL INTERVENTION MICROBIOLOGY AND ANTIMICROBIAL TREATMENT The earliest description of the vegetative lesions of infective endocarditis (IE) has been attributed to Lazarus Riverius (1589-1655). 1 Later, Giovanni Lancisi (1654-1720) provided a more complete description of these pathologic lesions of the heart in De Subitaneis Mortibus written in 1709. 2 Throughout the eighteenth and early nineteenth centuries there were many descriptions of endocarditis by investigators such as Morgagni and Corvisart, yet it was not until the middle to late 19th century that a link was made among the lesions, the associated inflammation, and the sequelae of the disease. In 1841, Bouillard (1796-1881) made the important connection between the inflamed endocardium, a “typhoid” state, and “gangrenous endocarditis.” This event was followed by the observations of Virchow (1821-1902), in 1847, and Kirkes (1823-1864), in 1852, connecting the dots between the presence of vegetative lesions and embolic events. 1 In his famous 1885 Gulstonian lectures Sir William Osler summarized the knowledge at that time and in addition made several important observations. First he described the acute and fulminating forms of the disease and was able to articulate specific characteristics of a more chronic and insidious form. He then improved the nomenclature of the disease and suggested calling the clinical course of the disease either “simple” or “malignant.”3,4 In addition, he described the classic presentation of a typical case and noted the diagnostic uncertainty in many cases. Finally, Osler believed that endocarditis would turn out to be a “mycotic” process, describing it as, “in all its forms, an essentially mycotic process; the local and constitutional effects being produced by the growth on valves, and the transference to distant parts of microbes, which vary in character with the disease in which it develops.” Since the days of Osler there have been many advances in our understanding of IE from pathophysiology to diagnosis, prognosis, and treatment; yet our knowledge remains remarkably incomplete. What is clear is that IE was, and remains, a serious and dynamic disease process. Over the past 30 years the incidence has remained relatively unchanged, and the associated mortality remains between 10% and 30% (depending on the organism, noncardiac conditions of the patient, and whether a native or prosthetic valve is involved). 5 Guidelines regarding endocarditis prophylaxis and therapeutic approaches to treatment have been now published and are the focus of this review. The true incidence of IE is difficult to ascertain. In a Swedish urban setting, Hogevik et al 6 found an incidence of 5.9 episodes per 100,000 person-years from 1984 to 1988. During a similar period in a Philadelphia metropolitan study, the total incidence was calculated to be 9.29 episodes per 100,000 person-years. 7 When intravenous drug users were excluded, this incidence fell to 5.02 episodes per 100,000 person-years. In both urban and rural settings in France, the incidence was estimated to be around 2.43 episodes per 100,000 person-years in 1991 8 and increased to 3.1 episodes per 100,000 person-years in 1999 9 with a peak incidence of 14.5 episodes per 100,000 person-years in the elderly. The growing incidence in elderly individuals has been confirmed in the Medicare population in the United States, in which it was 20.4 episodes per 100,000 person-years in 1998 (a 13.7% increase from 1986). 10 In fact, more than half of all cases of IE in the United States and Europe now occur in patients older than 60 years, and the median age of patients has increased steadily during the past 40 years. 11 Health care–associated IE results from health care–associated bacteremias. They include both nosocomial and nonnosocomial infections, have a high mortality rate, and are frequent in patients who are undergoing hemodialysis and/or who have other debilitating diseases. The typical patient nowadays is therefore less likely to be one with poor dentition and rheumatic disease 12 and more likely to be elderly and to have undergone a procedure to implant a device such as a prosthetic valve, pacemaker, and/or defibrillator 5 or to have a major comorbid condition. Table 25-1 summarizes cardiac conditions and the subsequent estimated incidence of IE per 100,000 patient-years. 13 Sex and age also influence the incidence of IE, with males predominating. Male : female ratios have been noted to range from 3.2 : 1 to 9 : 1.11,14 Of interest, 50% to 70% of children younger than 2 years in whom IE develops have no apparent underlying heart disease, whereas older children usually have a congenital heart condition. 15 Endocarditis in patients with injection drug use (IDU)—defined as the intravenous injection of recreational drugs such as heroin, cocaine, and amphetamines—also may occur when there is no apparent underlying valvular pathologic lesions. 16 Despite these exceptions, most patients do have identifiable underlying structural heart disease at the time of their endocarditis diagnosis.17,18 Earlier reports, before 1967, showed that rheumatic heart disease was the most common cardiac abnormality, being present in 39% of patients, 19 whereas later series suggest its presence in only about 6%. 18 Estimates of specific valvular lesion involvement is summarized in Table 25-2. TABLE 25-1 Estimated Incidence of Endocarditis Modified from Pallasch TJ. Antibiotic prophylaxis: problems in paradise. Dent Clin North Am 2003;47:665-679. TABLE 25-2 Estimated Predisposing Valvular Lesions in Patients with Endocarditis Despite advances in the diagnosis and management of IE, it remains a disease with unacceptably high morbidity and mortality. More than 50% of patients with IE have some type of serious complication, including HF, stroke, and paravalvular extension, whereas the in-hospital mortality rates (15% to 20%) and 1-year mortality rates (30% to 40%) have changed little over the past 20 years.5,9,20–22 Death is still disturbingly frequent and usually relates to cardiogenic shock, multiorgan failure, or stroke. Surgery is necessary and important for survival in around half the cases. 23 When valvular disease is considered as a whole, endocarditis still is an uncommon disease process. In the Euro Heart Survey 24 of the incidence of valvular disease in a general population, endocarditis was the major diagnosis in less than 1% of patients who were found to have aortic or mitral stenosis, in only 7.5% of those with aortic regurgitation, and in 3.5% of those who had mitral regurgitation. Left-sided native valve endocarditis (NVE) remains the most common presentation, accounting for 70% of all cases of IE. Mortality depends on comorbidities but still is at least 15% as a whole. 25 Degenerative mitral valve disease (mitral valve prolapse) is the leading predisposing valve lesion, with the risk particularly high in children and in patients older than 50 years. Patients with degenerative aortic valve disease are also at risk, helping explain the rising age of patients presenting with IE. One review estimated a slightly greater incidence of mitral than aortic involvement, 8% involving both, 4% involving the tricuspid valve, and 3.5% occurring in patients with congenital heart disease. 26 Endocarditis is unusual in patients with isolated pulmonary stenosis, atrial septal defect, mitral stenosis, or hypertrophic cardiomyopathy. Prosthetic valve endocarditis (PVE) accounts for up to 20% of the patients with endocarditis reported in a recent series from the International Collaboration on Endocarditis-Prospective Cohort Study. 27 Staphylococcus aureus was the most common organism. Having a prosthetic heart valve is the greatest risk for development of IE in every series. It is estimated that IE will develop in 1.4% to 3.1% of all patients with prosthetic valve at 1 year and 3% to 5.7% at 5 years. 28 There are two disparate risk periods for the development of PVE, an early period and a late period, although some writers believe it is more useful to consider early (2 months), middle (2 to 12 months), and late (>12 months) periods as the organisms involved shift gradually rather than abruptly. 28 The early period is generally defined as the first 60 days after heart surgery, and most of the implicated organisms are considered nosocomial. The late period involves organisms more like those involved in NVE. Although there has always been a suggestion that the mechanical valves are more susceptible to IE, by 5 years there appears to be no real difference, and most series do not suggest a difference in the risk by model, position, or type of valve (mechanical or bioprosthetic). 29 Some patient factors have been associated with PVE, including renal dysfunction, young age, prior endocarditis, and perioperative wound infections. 30 Health care–associated prosthetic valve endocarditis is identified in 36.5% of all cases, and most infections (71%) occur in the first year after the valve was implanted. The rate of in-hospital death remains high, at 23%, and its occurrence is associated with older age and the complications related to the surgical intervention. The higher risk of “redo” aortic valve replacement (AVR) for endocarditis is emphasized in a report of 313 patients by Leontyev et al. 31 Perioperative mortality was 24.3% in “redo” AVR for IE compared with 6.8% for redo AVR for reasons other than endocarditis. Right-sided endocarditis is seen in about 5% to 10% of IE surveys16,25 and has a better prognosis than left-sided disease, though the mortality remains high in patients with human immunodeficiency virus (HIV). 32 Right-sided endocarditis typically occurs in patients with illicit IDU (including patients with HIV) and those with structural abnormalities of the right heart due to congenital heart disease, pacemaker or defibrillator implantation, or central venous catheters. The most significant risk factor for right-sided IE is certainly IDU; however, left-sided disease may be more common in some groups of addicts. In one series, left-sided involvement occurred in 57% of patients with IDU compared with 40% with right-sided disease. 33 The most common infecting organism in patients with IDU is S. aureus, where it has been reported as the offending organism in up to 82%. 34 Prognosis in patients with IDU and IE is generally better than that in overall patients with IE who do not have a history of IDU, because of the lower risk of IE on the right side. 32 The patients with IDU and IE generally are also much younger than other IE populations. Of importance, the presence of HIV infection does not appear to alter the diagnostic use of the Duke criteria or the course of the disease, 35 although patients with a very low CD4+ count (<200 cells/mm3) are at greater risk. 9 The expanded use of cardiovascular electronic devices has resulted in infections not only on the device leads themselves but also on the tricuspid leaflet. A pocket infection appears to predispose to this form of IE. 36 A study from the Medicare database indicates that although the device implantation rate rose 42% in the 1990s, the IE infection rate rose 124%. 36 One estimate of the rate of these device-related infections suggests it is about 0.55 cases per 1,000 implants. 37 Removal of the device is almost always required for cure.38,39 A summary of a variety of noncardiac clinical conditions predisposing to IE and the organisms frequently associated with these conditions is given in Table 25-3. TABLE 25-3 Epidemiologic Factors Associated with the Development of Infective Endocarditis and the Commonly Associated Organisms Modified from Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. Circulation 2005;111:e394-e434. IDU is clearly a risk factor for IE, and those who use cocaine may have the greatest risk. 40 A prior history of endocarditis is an important predisposing factor in recurrent IE in patients with IDU. Recurrent endocarditis occurred in 4.5% of one large cohort of nonaddicts who survived their initial episode. 41 Pacemaker-associated infections have increased with the increased use of electrophysiologic (EP) devices. A report from the Multicenter Electrophysiologic Device Infection Cohort (MEDIC) registry 42 from 2009 through 2011 found that early (<6 months after implantation) device infections were generally related to pocket infections and that later IE was the result of other bacteremias. Staphylococci (coagulase-negative, methicillin-resistant, and methicillin-sensitive) were the most common organisms involved. As mentioned, effective treatment almost always requires removal of leads. Patients undergoing hemodialysis are the largest subgroup with health care–related IE.43,44 Predisposing factors in this population include intravascular access, calcific valvular disease, and impairment of the immune system. Of patients with health care–associated IE who are not undergoing dialysis, most have underlying predisposing conditions, including diabetes, cancer, and immunosuppressive therapy use. Identifiable underlying cardiac predisposition to IE occurs in less than 50% of this group. 5 Most invasive organisms originate from the skin or urinary tract, and the presence of intravenous lines or other invasive procedures is frequently evident. 45 Staphylococcus is the predominant offender. Other noncardiac conditions also can predispose to IE and are often associated with specific infecting organisms. For instance, in the International Collaboration on Endocarditis-Prospective Cohort Study, patients with IE due to S. aureus were significantly more likely to be hemodialysis dependent, to have diabetes, to have a presumed intravascular device source, to receive vancomycin, to be infected with methicillin-resistant S. aureus (MRSA), and/or to have persistent bacteremia. 46 Nosocomial endocarditis infections are also becoming more common. They are defined as a diagnosis of IE made more than 72 hours after admission in patients with no evidence of IE on admission or as development of IE within 60 days of a prior hospital admission during which there was risk for bacteremia or IE. 47 Nosocomial IE is usually a complication of bacteremia caused by an invasive intravascular procedure or an intravenous catheter–related device infection.47,48 It accounts for almost 10% of cases of IE in some series. A number of cases of IE have been reported in patients with HIV infection.49,50 Some valves have been infected with unusual organisms such as Salmonella and Listeria. 50 It has been reported that HIV infection is an independent risk factor for IE in injection drug users, 51 although this finding has not been confirmed in other studies. 50 The clinical examination, the organism involved and its response to therapy, and the echocardiographic information can establish the prognosis and guide decision making in the treatment of IE. A number of studies have examined other factors in an effort to understand prognosis in patients with IE. Chu et al 52 examined 267 consecutive patients with acute IE to determine factors early in the course of the disease that were independently associated with mortality. After controlling data for severity of illness with Acute Physiology and Chronic Health Evaluation (APACHE) II scoring, they found that the independent predictors of early mortality were the presence of diabetes mellitus (odds ratio [OR], 2.48; 95% confidence interval [CI], 1.24-4.96), S. aureus infection (OR, 2.06; 95% CI, 1.01-4.20), and an embolic event (OR, 2.79; 95% CI, 1.15-6.80). In a similar fashion, Hasbun et al 53 found that five baseline features were independently associated with mortality and developed a scoring system that included the following: mental status, lethargy or disorientation (4 points); Charlson comorbidity scale, 2 or greater (3 points); HF, moderate to severe (3 points); microbiology, S. aureus (6 points), other non-viridans infection (8 points); and therapy, medical therapy only (5 points). On the basis of this point system, patients with a score of 6 points or less only had 6% mortality at 6 months, whereas patients with a score of more than 15 points had 63% mortality. In other studies, the need for hemodialysis has also been found to portend a poor outcome,43,54 as has the presence of poor ventricular function. 55 Another study identified patients with an altered mental state, those with mobile vegetations, and those undergoing hemodialysis as the cohort with the highest risk. 56 Newer guidelines for endocarditis prophylaxis have created a great deal of controversy. In a population-based case-control study from Philadelphia, pulmonary, cardiac, gastrointestinal, and genitourinary procedures or surgery did not emerge as risk factors for the development of community-acquired endocarditis, and dental flossing reduced the risk only modestly. 57 One review emphasized that despite the known association of endocarditis with poor dental hygiene and a visit to the dentist’s office, the actual risk of endocarditis from a dental procedure (such as a tooth extraction) is exceedingly low 13 ( Table 25-4). In fact the event rate is so low, even in supposedly high-risk patients, that a trial designed to demonstrate an increased risk of IE from a dental procedure would require a prohibitively large number of patients and is likely not feasible. TABLE 25-4 Absolute Risks for Development of Infective Endocarditis from a Dental Procedure Modified from Pallasch TJ. Antibiotic prophylaxis: problems in paradise. Dent Clin North Am 2003;47:665-679. As part of the rationale eliminating most scenarios requiring endocarditis prophylaxis, both the American Heart Association (AHA) 58 and the British Cardiac Society 59 have now published guidelines suggesting its use only in patients who not only have the greatest risk for endocarditis but who also would suffer the most from the consequences of the disease. The two lists of such patients differ slightly. The AHA/American College of Cardiology (ACC) Adult Congenital Heart Disease guidelines also weighed in by suggesting that endocarditis prophylaxis be extended to coverage of the high-risk group during vaginal delivery. 60 The United Kingdom National Institute for Health and Clinical Excellence (NICE) working group has now taken the final step in this evolving process and suggests eliminating all prophylaxis before any procedure. 61 The NICE guidelines acknowledge that certain conditions predisposing to endocarditis—acquired valvular heart disease, valve replacement, structural congenital disease (including surgically corrected or palliated structural conditions, fully repaired ventricular septal defect or patent ductus arteriosus, and closure devices), previous endocarditis, and hypertrophic cardiomyopathy—may increase the risk of IE should bacteremia occur. On the basis of their review of all available data, the NICE guidelines working group recommends eliminating antibiotic prophylaxis for all dental or nondental procedures. They also note no preventive advantage of chlorhexidine mouthwash. Although there remain questions as to the value of eliminating endocarditis prophylaxis entirely, 62 most physicians have gradually accepted the current endocarditis prophylaxis guidelines with some trepidation.63–66 A comparison of the various recommendations from these guideline committees is summarized in Table 25-5. TABLE 25-5 Conditions That Pose Greatest Risk for Infective Endocarditis: Comparison of the American Heart Association and the British Cardiac Society Recommendations for Endocarditis Prophylaxis Therapy Following Dental Procedures * *Note that the American College of Cardiology (ACC)/American Heart Association (AHA) Guidelines for the Management of Adults With Congenital Heart Disease also suggest coverage of the high-risk patient during vaginal delivery 60 and that the National Institute for Health and Care Excellence (NICE) guidelines recommend no antibiotic coverage in any situation. 61 The normal heart valve is a three-layer histologic structure of endothelium, spongiosa, and ventricularis. Its endothelium is in continuity with the endothelium over the arterial, atrial, and ventricular walls. The endothelial lining is resistant to infection by bacteria and fungi except for a few highly virulent organisms. Events that result in endocarditis constitute a complex interaction between the host and the invading microorganisms and involve the vascular endothelium, the host immune system, hemostatic mechanisms, cardiac anatomic characteristics, surface properties, enzyme and toxin production by the microorganisms, and peripheral events that have caused the bacteremia. 28 Endothelial damage is the inciting event, followed by a platelet-fibrin deposition that provides a milieu for bacterial colonization. The role of endothelial damage as the inciting event is supported by the observation that the most likely areas of vegetation formation are similar to those where blood flow injury is most likely to occur: on the ventricular side of the semilunar valves and the atrial side of atrioventricular valves. 67 Jet lesions from insufficient valves may also damage endothelium, and vegetations may form on such sites of injury, for example, the mitral chordae in aortic regurgitation, the atrial wall (McCallum patch) in mitral regurgitation, and the septal leaflet of the tricuspid valve in a ventricular septal defect. Figure 25-1 illustrates the classic locations of endocardial and valvular lesions as well as the vegetation formation. FIGURE 25-1 Pathogenesis of infective endocarditis. A critical component in the formation of the infected vegetation is adherence of organisms to the endothelium or to the NBTE lesion. This adherence is facilitated by adhesive surface matrix molecules on the microorganism. Certain organisms appear to possess these surface molecules more than others, a difference that may explain their particular affinity for the NBTE lesion. For instance, streptococci that produce surface glucans and dextran appear to be more likely to cause endocarditis than those that do not. 68 It is undoubtedly no accident that the most common pathogens are gram-positive bacteria and enterococci, because these organisms not only have the greatest ability to adhere and colonize these initial lesions but also have multiple identifiable surface adhesins, sometimes referred to collectively as MSCRAMMS (microbial surface components reacting with adhesive matrix molecules). 69 For example S. aureus possesses clumping factor A (or fibrinogen-binding protein A) and fibronectin-binding protein A, both of which are known to be involved in valve colonization and invasion. The clumping factor appears to mediate the primary attachment of the bacteria to the NBTE lesion, and this step is followed by internalization of the organism, which is promoted by fibronectin-binding protein. Eventually proinflammatory and procoagulant responses occur. 70 Once safely inside the cells, the bacteria can survive, protected from antibiotics and host defense. 71 This process may explain why certain organisms, such as staphylococcal species and streptococci, which have the ability to bind to platelets and incite the clotting mechanism, may be more virulent than those organisms that are more readily shed into the bloodstream. The fact that S. aureus may also induce endothelial cells to produce a clotting tissue factor could, at least partially, explain why S. aureus adheres to relatively normal valve tissue. Particulate material that may be injected by intravenous drug users may also promote S. aureus adherence by stimulating adhesive binding molecules on normal heart valves. 72 This concept has been postulated to explain the distinct predilection for tricuspid valve involvement in intravenous drug users. 73 A potential therapeutic approach to prevent this binding was attempted by use of the St. Jude Silzon prosthetic valve ring, a silver-coated polyester ring (St. Jude Medical, Inc., St. Paul, Minnesota). Unfortunately, concerns regarding increased paravalvular regurgitation and emboli led to the product’s early withdrawal from clinical trials. 74 Enterococcus faecalis and other enterococcal species are also equipped with collagen adhesions 75 and aggregation substances 75 and are capable of biofilm production. 76 The clinical importance of biofilm production by these organisms has been strongly implicated in their antibiotic resistance and provides a potential therapeutic target for attacking the infections. 76 Some writers have postulated that local inflammation from degenerative lesions might have a direct role in endothelial infection. 5 Inflammatory mechanisms could potentially play a role in the pathogenesis of certain fastidious infections involving pathogens, such as Coxiella burnetii (Q fever), Chlamydia spp., Legionella spp., and Bartonella spp. 77 In up to 30% of patients, a preexisting cardiac abnormality may not be evident. 68 Several organisms appear capable of infecting apparently normal valves, including S. aureus, some streptococci, Salmonella, Rickettsia, Borrelia, and Candida spp. In addition to the mechanisms already described, it has even been postulated that some endothelial cells may contain metabolically latent organisms that eventually damage the endothelium. 78 The role of transient bacteremia in vegetation formation is indisputable. 79 Transient bacteremia is unavoidable, however, and occurs even during such mundane activities as chewing food and toothbrushing. Toothbrushing twice a day for a year has been found to result in a 154,000 times greater risk of bacteremia than would result from a single tooth extraction, the dental procedure associated with the highest bacteremia. 80 Taken overall, the cumulative exposure from such routine activities may be as high as 5.6 million times greater than that derived from a single tooth extraction. 80 Data such as these have led to the skepticism regarding the value of endocarditis prophylaxis during dental procedures. Host defenses against infection likely also play an important but poorly defined role. Perhaps surprisingly, IE is not more prevalent in immunocompromised patients, with the possible exception of those with HIV disease. 81 Endocarditis involving gram-positive organisms is much more common than gram-negative, in part because of differences in the organism itself, but possibly also related to host defenses. For instance, the C5b-C9 membrane-attack complex of complement has a much greater killing effect on the membranes of gram-negative than of gram-positive organisms. 5 Platelet microbicidal proteins may also play some role, 82 especially because platelet deposition is so important in in vegetation formation. The role of viruses as causative agents in IE remains unproven. A 2011 report revealing coxsackievirus cultured from an infected intracardiac patch raises the issue anew. 83 Burch et al 84 have suggested the possibility for many years, though later reviews of culture-negative endocarditis have not provided much evidence for viral etiologies. 85 All of these interacting processes eventually lead to proliferation of the infecting organism within the vegetation. The cycle of adherence, organism growth, and platelet-fibrin deposition is then repeated again and again as the vegetation grows and develops. After treatment, capillaries and fibroblasts may appear in the lesion, but untreated lesions tend to be avascular. Necrosis with various stages of healing may occur along with vasculitic components in the healed lesion. Even after successful antimicrobial therapy, many sterile vegetation masses persist indefinitely. 86 The prevalences of the clinical features observed in patients with IE are summarized in Table 25-6. Many of these features were espoused by Osler in the Gulstonian lectures4,87 but are rarely seen in an era when diagnostic testing is better and there is antimicrobial therapy. Most patients continue to have an initial indolent course from 2 weeks to many months with vague symptoms. Symptoms include fever, chills, anorexia, weight loss, night sweats, and malaise. Fever is the most common symptom, occurring in from 64% to 93% of patients with NVE, 85% with PVE, and 75% to 88% of patients with IDU and IE. It is less common in elderly patients and in patients with HF, renal failure, severe debility, or previous antibiotic therapy. 88 Persistent fever more than 1 week after therapy requires further investigation into its cause (e.g., an abscess somewhere), a nosocomial infection, drug fever, or inadequate IE therapy. A murmur is apparent in 80% to 85% of patients, 28 although auscultation is a dying art in cardiology and a murmur may not always be recognized by health care providers even when present. The murmur of acute and fulminant aortic regurgitation may be particularly difficult to hear because there is little diastolic gradient. Whereas tricuspid regurgitation should be evident from examination of the jugular venous pulse, the murmur is often quite soft when right ventricular systolic pressure is normal. A variety of peripheral cutaneous manifestations highlight the classic endocarditis examination ( Figure 25-2). Unfortunately many of these peripheral stigmata are rare today. Emboli can be observed in many areas, such as mouth and conjunctival petechiae, nail bed splinters, skin Janeway lesions, Osler nodes, and Roth spots. Splinter hemorrhages tend to occur in the proximal half of the nail bed, as opposed to splinters due to trauma, which occur in the outer half. Janeway lesions are painless, erythematous skin lesions that often appear in crops on the hands or feet. They represent embolic events similar to those in splinters. Biopsies reveal that they are microabscesses without arteritis, and organisms can often be cultured from them. Osler nodes are painful lesions that manifest as nodules on the pads of the toes or the fingertips and may persist for days. The cause for Osler nodes is unclear, but the fact that they may be seen in other settings, such as systemic lupus erythematosus (and there is histologic evidence for perivasculitis on biopsy), has led many to regard them as immunologic phenomena. Rarely, organisms have been cultured from Osler nodes, suggesting that emboli may at least be the inciting mechanism. 89 Roth spots are retinal hemorrhages with a white center. They most likely represent septic emboli, but like Osler nodes, they have been described in other clinical settings and especially as a manifestation of systemic lupus erythematosus, anemia, diabetes, multiple myeloma, and HIV infection. 90 Frank retinal artery occlusion may also occur. Musculoskeletal aches and pains are common in IE and often occur early in the course. 91 Any joint can be involved, but back and shoulder pains are most frequently cited. 92 Septic emboli may result in osteomyelitis or bone abscess formation (especially in the spine). Musculoskeletal pain must be taken seriously if it persists during the course of therapy. Neurologic symptoms are common, being seen in as many as 30% to 50% of patients with IE. Symptoms appear to be more common in patients with IDU and those with staphylococcal IE. 93 Embolic stroke is the most common and serious manifestation. Intracranial hemorrhage may occur from a ruptured arterial vessel, a ruptured mycotic aneurysm, or bleeding into a thrombotic stroke distribution. 94 In addition, neurologic symptoms may be related to cerebritis or meningitis or to toxic or immune-mediated injury. Brain abscess is rare, but microabscesses from virulent organisms, such as S. aureus, occur with some frequency. 95 Meningitis may be a major feature in IE due to Streptococcus pneumoniae. Splenic emboli are probably underreported. In the preantibiotic era, splenomegaly was common. Now about 25% to 50% of patients may have evidence of enlarged spleen. Autopsy series suggest that splenic infarcts are often present without clinical symptoms. 96 The diagnosis of IE hinges on clinical suspicion and the demonstration of continuous bacteremia. It was not until the late 1970s that Pelletier and Petersdorf 97 developed a case definition based on a 30-year experience of caring for patients with IE in Seattle. Although this definition was highly specific, it lacked sufficient sensitivity. In 1981, von Reyn et al 98 published an analysis that provided four diagnostic categories for cases of suspected IE (rejected, possible, probable, and definite), and the effort improved both the sensitivity and the specificity of the previous case definition. The definition did not incorporate imaging information, however. In 1994, Durack et al 99 from Duke University Medical Center incorporated echocardiography into the criteria for the first time, giving rise to what have come to be known as the Duke criteria. These criteria have been validated subsequently by many other studies,100–102 including the latest modifications ( Table 25-7).103,104 There are now three diagnostic categories. Definite endocarditis is considered to be present if there is pathologic evidence (surgical pathologic histology or culture or vegetation histology or culture) or if there is clinical evidence as demonstrated by the presence of two major criteria or one major criterion and three minor criteria or five minor criteria. Possible endocarditis is defined as having one major criterion and one minor criterion or three minor criteria. Rejected diagnosis is defined as having a firm alternative diagnosis or sustained resolution of the evidence for endocarditis after 4 or fewer days of antibiotic therapy or no pathologic evidence of endocarditis at surgery or autopsy after 4 or fewer days of therapy. TABLE 25-7 The Modified Duke Criteria for Diagnosis of Infective Endocarditis * I. Major Criteria
Infective Endocarditis
Historical Background
Epidemiology
Overall Incidence
Incidence of Infective Endocarditis and Associated Mortality
PERCENTAGE OF ENDOCARDITIS CASES AFFECTED
Native Valve Disease
Left-sided:
70
Mitral regurgitation
21-33
Aortic regurgitation
17-30
Aortic stenosis
10-18
Congenital heart disease:
4-18
Cyanotic heart disease
8
Tetralogy of Fallot
2
Ventricular septal defect
1.5
Patent ductus arteriosus
1.5
Eisenmenger syndrome
1.2
Atrial septal defect, coarctation of aorta
<1
Right-sided (including device infection)
5-10
Prosthetic Valve
20
Groups at High Risk for Development of Infective Endocarditis, and the Determination of Prognosis
EPIDEMIOLOGIC FEATURE
COMMON MICROORGANISM(S)
Injection drug usage
Staphylococcus aureus, coagulase-negative staphylococci, β-hemolytic streptococci, fungi, aerobic gram-negative bacilli (including Pseudomonas), polymicrobial
Indwelling medical devices
S. aureus, coagulase-negative staphylococci, β-hemolytic streptococci, fungi, aerobic gram-negative bacilli, Corynebacterium spp.
Poor dental health
Viridans group streptococci, HACEK group (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella), nutritionally deficient streptococci, Abiotrophia defectiva, Granulicatella spp., Gemella spp.
Diabetes mellitus
S. aureus, β-hemolytic streptococci, Streptococcus pneumoniae
Acquired immunodeficiency syndrome
Salmonella spp., S. pneumoniae, S. aureus
Chronic skin infections, burns
S. aureus, β-hemolytic streptococci, aerobic gram-negative bacilli, fungi
Genitourinary infections or manipulation, including pregnancy, abortion, and delivery
Enterococcus spp., group B streptococci, Listeria monocytogenes, aerobic gram-negative bacilli, Neisseria gonorrhoeae
Alcoholic cirrhosis
Bartonella spp., Aeromonas spp., Listeria spp., S. pneumoniae, β-hemolytic streptococci
Gastrointestinal lesions
Streptococcus bovis, Enterococcus spp., Clostridium septicum
Solid organ transplantation
S. aureus, Aspergillus fumigatus, Candida spp., Enterococcus spp.
Homelessness, body lice
Bartonella spp.
Pneumonia, meningitis
S. pneumoniae
Contact with containerized milk or infected farm animals
Brucella spp., Pasteurella spp., Coxiella burnetii, Erysipelothrix spp.
Dog/cat exposure
Bartonella spp., Pasteurella spp., C. septicum
Prophylaxis
RISK
General population
1 per 14 million procedures
Patients with:
Mitral valve prolapse
1 per 1.1 million
Congenital heart disease
1 per 475,000
Rheumatic heart disease
1 per 142,000
Prosthetic valve
1 per 114,000
Prior endocarditis
1 per 95,000
Pathophysiology and Pathogenesis
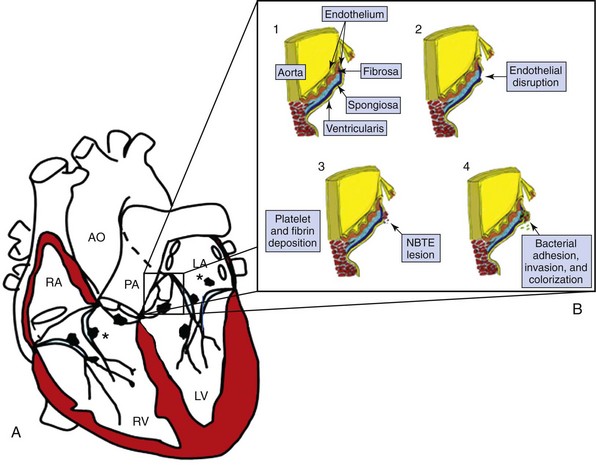
A, Sites of high-velocity jets where endocarditis vegetations occur. Note that these are on the atrial side of an atrioventricular valve and on the ventricular side of a semilunar valve. In addition, jet lesions from semilunar valves can result in lesions on chordae. Asterisk marks areas of jet lesions (McCallum patches) on endocardium from lesions such as a ventricular septal defect (on tricuspid septal leaflet) or on the left atrium from a mitral regurgitation jet. B, The steps in the development of the endocarditis lesion on the aortic valve. (1) The normal aortic valve leaflet. A thickened portion below the commissural line is the area where the leaflets coapt and trauma is most likely to occur. Endothelium covers the valve and is an extension of aortic and ventricular endothelium. The fibrosa provides major support for the leaflet. The ventricularis underlies the free edge, and the spongiosa lies between the two in the central portion. (2) The initial insult with endothelial injury and exposure of valve collagen. (3) Platelet and fibrin deposition with the formation of the nonbacterial thrombotic endocardial (NBTE) lesion. (4) Adhesion of microorganisms and then invasion into the NBTE lesion followed by colonization. Inflammatory cells become evident, elastin and collagen disruption occurs, and the valve destruction begins. AO, Aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle. (Adapted from Bashore TM, Cabell C, Fowler V Jr. Update on infective endocarditis. Curr Probl Cardiol 2006;31:274-352.)
Diagnosis
Clinical Manifestations
The Evolution of the Duke Criteria
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Infective Endocarditis
Only gold members can continue reading. Log In or Register to continue