Infected Aortofemoral Bypass Limb with Pseudoaneurysm (with Local Flap Coverage)
CYNTHIA K. SHORTELL and KATHARINE L. MCGINIGLE
Presentation
A 60-year-old male smoker underwent aortobifemoral bypass with a bifurcated Dacron graft for claudication due to aortoiliac occlusion. Three weeks later at the postoperative visit, his claudication had resolved, but he reported low-grade fevers for the last 2 to 3 days accompanied by new tenderness at his right groin surgical site. In clinic, he was afebrile with stable vital signs. Cardiopulmonary exam findings were normal, his abdomen was soft with a well-healed laparotomy incision, and his left groin incision was well healed, but his right groin incision had a punctate area of breakdown with turbid drainage (Fig. 1). The remainder of the incision was healed, and there was no cellulitis. He had palpable femoral and pedal pulses. His white blood cell (WBC) count was 9000/mm3, his chemistry panel was within normal limits with the exception of an albumin of 2.7 g/dL, and blood and urine cultures were negative.
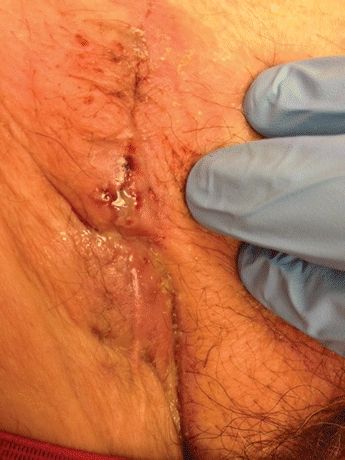
FIGURE 1 Photograph of right groin following aortobifemoral bypass with a punctate area of breakdown with turbid drainage. The remainder of the incision was healed, and there was no cellulitis.
Differential Diagnosis
In this patient with a history of graft placement and new drainage from his right groin incision, superficial surgical site infection (SSI) and prosthetic graft infection should be at the top of the differential diagnosis. The incidence of prosthetic graft infection in aortofemoral bypasses is 0.5% to 3%.
Pseudoaneurysm, infected or sterile, must also be considered in any patient with known vascular anastomosis, especially if there is a prominent femoral pulse or pulsatile mass that is tender to palpation. This diagnosis can be catastrophic if missed. An incidence of pseudoaneurysms in up to 13% of femoral arterial anastomoses (most commonly in aortofemoral grafts) has been reported. Pseudoaneurysm is more likely to occur if there is graft infection. Late presentation is common, and the most likely etiology is degenerative changes in the patient’s arterial wall with dehiscence of the suture line. Careful evaluation of both groins is necessary because one third of the patients will have bilateral pseudoaneurysms.
Although this patient presented in the immediate postoperative period, many patients with graft infections present years after their surgery with a fever of unknown origin. Fever of unknown origin is most often the result of infections (tuberculosis, occult abscesses, endocarditis), connective tissue disease (vasculitis, lupus), or malignancy (lymphoma, leukemia). A clue suggesting graft infection may be a history of nonhealing foot wounds, contaminated surgical procedures, or dental or urologic procedures that may lead to a transient bacteremia causing seeding of the graft.
Workup
1. Wound culture and serum markers
If graft infection or infected pseudoaneurysm is suspected, then Gram stain and culture of the drainage from the wound (or ultrasound-guided sample of perigraft fluid) may be helpful. Often, late graft infections are caused by low-virulence organisms, and Gram stain may only show elevated levels of white cells and no bacteria. Nonspecific serum markers (ESR and CRP) may also be elevated in infection.
2. Ultrasound
Doppler ultrasound is a fast and reliable method of confirming the diagnosis of pseudoaneurysm and can also characterize the size and presence of thrombus (Fig. 2).
3. CT angiography
CTA can confirm the diagnosis of pseudoaneurysm (Fig. 3) and also better evaluate the flow lumen, presence of extravasation, presence of perigraft infection, and involvement of the intra-abdominal portion of the graft and contralateral limb. Findings suggestive of infection on CT include loss of normal tissue planes, fat stranding, lymphadenopathy, collections of gas or fluid, pseudoaneurysms (Fig. 3), retroperitoneal abscess, and vertebral osteomyelitis.
4. WBC scan
If diagnosis is still unclear after ultrasound and CT, then radionucleotide scintigraphy (indium- or technetium-labeled WBC scans) can identify areas of inflammation. These tests may be useful in identifying very low-grade graft infections in patients with nonspecific presentations; however, they have a high false-positive rate since hematomas and sterile inflammatory changes cannot be differentiated with these scans.
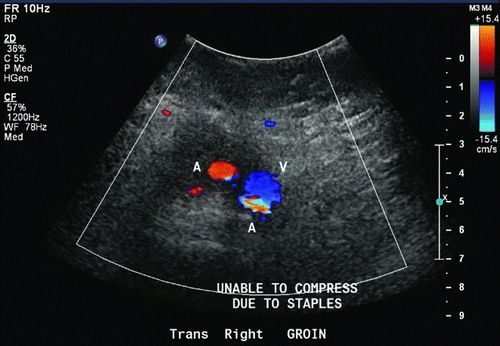
Figure 2 Ultrasound of right groin suggesting the presence of right femoral artery pseudoaneurysm.
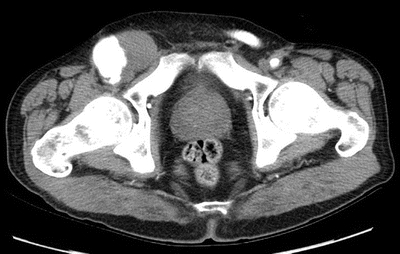
Figure 3 CTA documented right femoral artery pseudoaneurysm following aortobifemoral bypass.
Case Continued
Graft infection was suspected in this patient. Gram stain of the right groin drainage showed gram-positive bacteria. ESR was elevated at 30 mm/hour. Ultrasound confirmed the presence of a 5-cm femoral artery pseudoaneurysm in the right groin with active flow within the sack. CTA demonstrated widely patent flow lumen from the aorta into the bilateral extremities, and there was also contrast filling the 5-cm pseudoaneurysm. CT also revealed fluid, fat stranding, and patchy lymphadenopathy around the right limb of the graft in the groin. The abdominal portion and left limb appeared normal and were well incorporated with adjacent tissues, and no surrounding fluid was noted.
Diagnosis and Treatment
Based on the workup, early and localized aortofemoral graft infection with pseudoaneurysm was the confirmed diagnosis and the patient needed to be taken to the operating room. A pseudoaneurysm with active flow requires urgent intervention. While timeliness is important, careful operative planning is needed in the setting of graft infections since there are many variables like the type of organism, extent of infection, presence of systemic disease, and patient’s ability to heal that will determine the success of your intervention.
Overall, Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli are the causative organisms in 80% of graft infections. Less commonly involved organisms include Klebsiella, Proteus, Enterobacter, Pseudomonas, and nonhemolytic streptococci. The most likely etiology of early graft infection is contamination at the time of implantation. The pores of the graft material will harbor organisms indefinitely.
Early graft infections occur within 4 months, and the involved organisms are usually more virulent such as S. aureus or gram-negative organisms. Early infections are less common than are late infections and are usually associated with a postoperative complication such a cellulitis, SSI, or delayed wound healing.
Late graft infections present after 4 months, and the mean time of presentation is 6 years postoperatively. These infections are usually harder to detect as most of them are secondary to organisms with low virulence like S. epidermidis. Late infections are more likely to involve the intra-abdominal portion of the graft, and may also present with gastrointestinal bleeding via an enteric fistula. Due to the difficulty of making the diagnosis, these patients often have delay in definitive surgical management, and nutritional compromise and immunologic compromise can be significant factors, also.
Discussion
Gram-positive infections occurring in the early postoperative time period and localized to a single groin can be treated with a graft preservation strategy with a high expectation of success. The patient in this scenario was taken to the operating room for right groin exploration and debridement, primary repair of the pseudoaneurysm, and muscle flap coverage. The right groin incision was reopened with care not to disrupt the tissue around the pseudoaneurysm. There was scant fluid around the graft at the level of the pseudoaneurysm sack. To gain proximal and distal control, vessel loops were passed around the graft, superficial femoral artery, and deep femoral artery. The wound was copiously irrigated with normal saline, and all infected-appearing tissue was debrided. To repair the pseudoaneurysm, the anastomosis was divided and the graft and artery were debrided back to healthy tissue. Only minor debridement was necessary, and tension-free primary reanastomosis was performed without needing an interposition graft. In the setting of graft preservation, we closed the wound with a sartorius muscle flap.
Surgical Approach
The primary goals of the surgical procedure are to address the infected graft and to maintain blood flow to the extremity to preserve limb viability. Within this framework, there are many choices for excision and reconstruction. The first is whether an in situ reconstruction or an extra-anatomic bypass will be performed. Second, especially for in situ reconstruction, one must decide the type of conduit to use. Third, a decision as to whether or not complex wound closure (i.e., muscle flap) is required must be made.
Complete graft excision and revascularization with an extra-anatomic bypass in different tissue planes is the most definitive way to remove the source of infection. Despite the morbidity of abdominal reoperation, if the patient presents with systemic inflammatory response syndrome (SIRS) or sepsis or if there is known gram-negative infection, then complete graft excision and extra-anatomic revascularization is recommended. Complete graft excision is recommended for any graft infected with gram-negative bacteria whether early or late because these infections are more virulent, more likely to involve the whole graft, more likely to result in vascular deterioration (graft blowout), and less likely to respond to conservative treatment.
Most commonly, the extra-anatomic procedure performed is an axillobifemoral graft using ringed 6- or 8-mm polytetrafluoroethylene (PTFE). The sequence and timing of the stages of this operation is important and must be individualized to each patient. Performing an extra-anatomic reconstruction followed by immediate removal of the infected prosthesis increases the risk of seeding the new graft material with the same organism; however, it is generally the preferred approach. Removing the infected graft followed by reconstruction increases the ischemia time to the legs and may increase the risk of complications, such as compartment syndrome, paresis, or amputation.
In patients who are clinically stable with radiographic evidence of retroperitoneal or intra-abdominal infection and known gram-positive or low-virulence infections, complete graft excision is also recommended, but in situ revascularization is possible by creating a neoaortoiliac system (NAIS) using superficial femoropopliteal vein. The benefits of using in situ, autogenous vein are improved primary patency and less reinfection compared to prosthetic material. Performing a NAIS in a patient with sepsis, gram-negative, anaerobic, or fungal infection increases the odds of postoperative 30-day mortality, but has been done successfully by centers with extensive experience with the operation.
The patient in this clinical scenario had a localized infrainguinal infection with a gram-positive organism, negative blood cultures, and no signs of systemic illness, so a less aggressive operation was planned. Local excision and in situ revascularization using autogenous vein or prosthetic conduits has been described with a 10% recurrent infection rate. This option is also appropriate for patients with late infections with only extra-abdominal limbs of the bypass affected if there are no clinical signs of systemic infection. Often, these low-virulence late infections only have neutrophils on wound culture, but the excised graft is usually found to have coagulase-negative staphylococci.
In the case of axillofemoral PTFE bypass, it is critical to tunnel the conduit in a new plane away from the contaminated groin sites. Tunneling lateral to the sartorius muscle to approach the mid–deep femoral artery allows for anastomosis creation at a clean site. After graft excision from the groin sites, loosely closing the tissue over a drain and placing a vacuum-assisted wound therapy device are options to promote healing and minimize recurrent SSI. If contaminated sites have to be used, or in case of graft preservation, complex closure with a muscle flap can increase salvage rates. The muscle flap provides healthy, well-vascularized tissue to the area over the vascular graft and fills the soft tissue defect created by the debridement. Rectus femoris, sartorius, and gracilis rotational flaps have all been described as methods of durable coverage in acute graft infections. There is a 5% to 10% rate of persistent/recurrent infection reported after flap closure (Table 1).
TABLE 1. Infected Aortofemoral Bypass Limb with Pseudoaneurysm




