Infected Aortic Graft
SUKGU M. HAN, CHARLES M. EICHLER, and CHRISTOPHER D. OWENS
Based on the previous edition chapter written by Christopher Owens and Michael Belkin
Presentation
A 56-year-old man had previously undergone an aortobifemoral Dacron graft placement for claudication due to aortoiliac occlusive disease. In addition, concurrent left femoral to above-knee bypass graft with prosthetic graft was performed for infrainguinal peripheral vascular disease (PVD). He recovered uneventfully and was well until 2 months later when he presented with drainage from left groin incision along with intermittent fevers, fatigue, malaise, and weight loss. He is admitted to the hospital and placed on broad-spectrum intravenous antibiotics. Upon admission, he is noted to have temperature spikes to 102.4°F. His examination is notable for ill-appearing male with purulent drainage from the left groin. He has a well-healed abdominal incision and left groin incision; however, there is a small area of breakdown in the superior pole of his left groin incision with an apparent sinus tract with drainage, and a nontender, nonpulsatile mass is palpable under the draining sinus. Both of his lower extremities are warm and well perfused without ulcers or petechiae. Posterior tibial and dorsalis pedis arteries have multiphasic Doppler signals bilaterally. His white blood cell (WBC) count is 18,100/mm3, and his erythrocyte sedimentation rate is 64 mm/hour. His chemistry panel is normal with the exception that his albumin is 2.2 g/dL. A computed tomography (CT) scan is ordered.
Differential Diagnosis
A pseudoaneurysm must be considered in any patient with a prominent femoral pulse and a known anastomosis in the groin. Ultrasonographic examination is a fast, safe, and reliable method of confirming the diagnosis. The incidence of pseudoaneurysms is reported to be 1% to 5% in the literature, and the femoral anastomosis is most commonly involved in the setting of previous aortobifemoral bypass graft. The common thread among the numerous etiologic factors reported is degenerative changes in the host’s arterial wall with dehiscence of the suture line. Because the anastomosis will forever be dependent on the integrity of the suture line, strict adherence to basic vascular surgical principles, such as using nonabsorbable, monofilament suture and taking bites that include all layers of the arterial wall, is essential when sewing prosthetic material to native artery.
Any patient presenting with a pseudoaneurysm should compel the clinician to rule out graft infection. While infectious etiology has been reported in as many as 24% of femoral false aneurysms, pseudoaneurysms occur in approximately 25% of graft infections. Typically, pseudoaneurysms due to graft infections tend to occur earlier than noninfected cases. Furthermore, a pseudoaneurysm in one groin could also herald other graft problems, such as contralateral pseudoaneurysm, and mandates close scrutiny of the entire graft. Finally, a malnourished man presenting with fever of unknown origin and a pulsatile groin mass after aortobifemoral reconstruction has an infected graft until proven otherwise.
CT Scan
CT Scan Report
CT scan demonstrates a peripherally enhancing fluid collection, containing gas, around the left femoral anastomosis (Fig. 1). Superiorly, the fluid collection tracks around the left limb of the aortobifemoral bypass graft above the level of the inguinal ligament. Inferiorly, it is contiguous with the sinus tract out to the skin where purulent drainage was noted on physical examination.
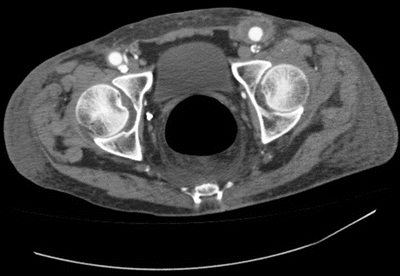
FIGURE 1 Preoperative CT scan showing a fluid collection over left limb of aortobifemoral bypass graft contiguous with the draining sinus in the groin.
Diagnosis and Recommendation
Findings on this patient’s CT scan along with the clinical exam confirm infection involving at least the left limb of the aortobifemoral graft. In general, findings suggestive of graft infection on contrast-enhanced CT include any of the following: loss of normal tissue planes and stranding in the retroperitoneal space, abnormal collections of fluid and gas, focal thickening of the bowel, false aneurysms, vertebral osteomyelitis, and juxta-aortic retroperitoneal abscess. Postoperative perigraft gas and hematoma are usually absorbed by 3 months. Although computed tomography’s diagnostic accuracy has certainly been validated in advanced-stage graft infections, it is inconsistent in low-grade infections with less virulent organisms.
Another modality that can be used in identifying patients with aortic graft infections who do not have obvious findings on the CT scan is imaging techniques using radionuclide scintigraphy. These include indium-labeled WBC, immunoglobin G, or technetium-labeled WBC scans. These methods identify graft infection by radioisotopic imaging of inflammatory sites. They are a useful adjunct in the nonspecific clinical presentation of low-grade graft infections; however, there have been false positives in patients with hematomas or sterile inflammatory processes around the graft. More recently, several fusion imaging techniques have been used to distinguish graft infection from sterile inflammatory response. Particularly, FDG-PET/CT looking at the uptake pattern of radioactive-labeled glucose has been shown to have high positive predictive value for graft infection.
Percutaneous localization of a perigraft cavity by the injection of contrast can be a useful aid in making the diagnosis, although there is a theoretical possibility that it can contaminate an otherwise sterile graft. Culture and Gram stain of the perigraft fluid can often identify the etiologic agent, but failure to do so does not rule out graft infection. A late graft infection, often from a low-virulence microorganism, may only yield WBCs on Gram stain.
Case Continued
The patient is taken to the operating room and undergoes an excision of the infected aortobifemoral and femoropopliteal bypass grafts, with an in situ aortofemoral reconstruction. Initially, the left limb of the aortobifemoral bypass graft is assessed through a small left flank incision. As suspected, the left limb is found to be poorly incorporated and bathed in a purulent fluid collection with Gram stain confirming multiple gram-positive organisms in the perigraft fluid. Bilateral femoral arteries are exposed through longitudinal groin incisions; then, the aorta is exposed through a bilateral subcostal incision. In this patient, with extensive adhesions in the infrarenal aorta, supraceliac exposure is performed with medial visceral rotation. The distal anastomoses are disconnected first, and the surrounding tissue is debrided. The remainder of the graft is removed with a supraceliac clamp. The native aorta is debrided until intact tissue is encountered. An in situ aortofemoral reconstruction is performed with a composite graft using a cryopreserved descending thoracic aorta spliced with a cryopreserved femoral vein. The proximal anastomosis is fashioned end to end, followed by the distal, end-to-side anastomosis to the left common femoral artery. Left to right femoral crossover bypass is constructed using an additional cryopreserved femoral vein (Fig. 2). Left femoral to popliteal bypass graft is completely excised through a medial distal thigh incision. Upon completion of the excision and the in situ reconstruction, the patient is found to have monophasic Doppler signals over the dorsalis pedis and posterior tibial arteries bilaterally. At this point, based on the facts that (1) the patient has undergone an extensive operation with 3.5 L of blood loss, requiring massive transfusion and resuscitation, and (2) left leg is not acutely ischemic after the excision of femoropopliteal vein, the decision is made not to perform a redo femoropopliteal bypass. The retroperitoneal wound is debrided and cultured. The explanted graft is cultured and yielded Staphylococcus epidermidis. Sartorius flaps are performed to provide additional soft tissue coverage over the new grafts in the bilateral redo groin incisions.
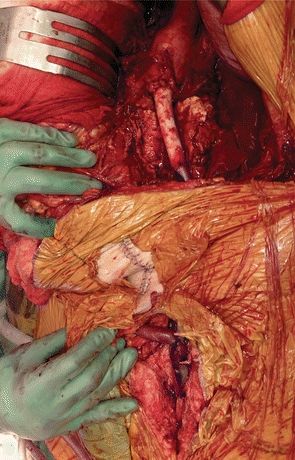
FIGURE 2 In situ reconstruction using cryopreserved conduit.



