Chapter 142
Infected Aneurysms
Adam W. Beck, Gabriela Velazquez-Ramirez
Based on a chapter in the seventh edition by Mitchell R. Weaver and Daniel J. Reddy
Despite significant advances in vascular technology over the last two decades, management of infected aneurysms remains one of the most challenging problems that modern surgeons face. A survey of the literature demonstrates reports of infected aneurysms in essentially every named vessel in the human body, often in challenging anatomic locations. Patients with infected aneurysms are often elderly and frail, with multiple medical comorbidities, which further increases the difficulties with their management. Unfortunately, medical treatment based on tailored antibiotic therapy alone is generally unsuccessful, and excision of the involved structures is often needed to eradicate infection. Early recognition with culture-directed antibiotic therapy and definitive surgical treatment is key to a good outcome. Historically, removal of the infected tissue, in combination with either in-situ or extra-anatomic reconstruction has been the mainstay of therapy. However, in recent years, scattered reports of primary treatment or bridging therapy with endovascular aneurysm exclusion have been reported with mixed results.
History and Epidemiology
Osler1 presented the first comprehensive discussion of infected aneurysms in 1885. He described infected peripheral aneurysms that occurred in patients with bacterial endocarditis,1 and described the pathogenesis as an embolism with bacteria-laden material from the heart that led to destruction of the arterial wall at the site of the embolus. Based on the eccentric saccular appearance of these infected aneurysms, he coined the term “mycotic” to describe their mushroom-like appearance. Unfortunately, this term led to confusion, with some assuming it applied to infections caused by fungi, and others applying the term to all infected aneurysms, rather than only those associated with bacterial endocarditis. For this reason, the term is best avoided, in favor of a more precise description. In 1923, Stengel and Wolfert2 described 4 patients, reviewed another 213, and found that 30 patients had no evidence of bacterial endocarditis. They demonstrated that infected aneurysms could result from a variety of septic conditions, without endocarditis. Sommerville et al,3 in 1959, reported on the existence of a third type of arterial infection—one that occurred in preexisting atherosclerotic aneurysms—and found 6 infected aneurysms in a series of more than 20,000 Mayo Clinic autopsies. Infected pseudoaneurysms have been described more recently, and are usually associated with intravenous or intra-arterial drug injection.
The overall incidence of arterial infections and infected aneurysms has increased in recent decades with the increasing prevalence of immunosuppressed patients,4–7 invasive hemodynamic monitoring,8 catheter-based procedures,9–11 and illicit drug use.12–16
Pathogenesis and Etiology
The term infected aneurysm includes multiple distinct conditions, which can be classified into four types based on etiology: (1) microbial arteritis with aneurysm formation due to noncardiac origin bacteremia or contiguous spread of a localized infection; (2) posttraumatic infected pseudoaneurysms, most commonly related to intra-arterial drug abuse; (3) infection of preexisting aneurysms from bacteremia or contiguous spread; and (4) infected (mycotic) aneurysms from septic emboli (as classically described by Osler1) (Table 142-1). The pathogenesis of an infected aneurysm is different depending on the type.
Microbial Arteritis
Bacterial seeding can occur in nonaneurysmal arteries with preexisting wall irregularities caused by conditions such as atherosclerosis or congenital abnormalities (e.g., aortic coarctation, patent ductus arteriosus).18,19 Further, normal arteries can become contiguously involved by local infection. Once a local arterial wall infection is established, suppuration, localized perforation, and pseudoaneurysm can result. Alternatively, more diffuse infection can result in rapid development of a true aneurysm, although these are often more eccentric than a typical degenerative aneurysm. All named arteries are at risk, but the aorta is the most commonly affected site, likely because it is the most frequent site of atherosclerosis and aneurysm formation.15,20–23
Infected aneurysms associated with immunosuppressed states may present with atypical clinical features. Conditions associated with arterial infection include diabetes, cirrhosis, chronic hemodialysis, posttransplant immunosupression, human immunodeficiency virus infection, alcoholism, chronic glucocorticoid therapy, chemotherapy, and malignancy.11,16–19,24–29 In their study of 43 patients with infected aneurysms, Oderich et al7 found 70% of patients were in an immuncompromised state.
Posttraumatic Infected Pseudoaneurysms
Arterial trauma leading to direct inoculation of the arterial wall has become an increasingly common mechanism by which infected aneurysms develop. Bacteria can be introduced at the time of arterial catheterization performed for monitoring, diagnostic, or therapeutic interventions, or during drug abuse with inadvertent or intentional intra-arterial injection. Notably, the use of percutaneous closure devices for endovascular procedures has also been reported to be associated with increased incidence of infected pseudoaneurysms.30,31 Almost all infected arterial aneurysms associated with this type of trauma are pseudoaneurysms in which the arterial wall continuity is lost. Not surprisingly, the common femoral artery is the most common location, but posttraumatic infected pseudoaneurysms involving the carotid, brachial, external iliac, and subclavian arteries have also been reported.10,24,25
Infection of Preexisting Aneurysms
Preexisting aneurysms can be secondarily infected by hematogenous or contiguous spread. Aneurysms are susceptible to infection because the diseased intima may allow bacterial seeding and deeper infection of the arterial wall.3 Interestingly, bacteria are not infrequently found in the thrombus associated with degenerative aneurysms without clinically apparent infection. Sommerville et al3 demonstrated bacteria in aortic thrombus in 3.4% of their patients. Bennett et al20 and Jarrett et al19 demonstrated similar findings and suggested that aneurysms with bacteria growing in the thrombus were more apt to rupture. Further, in a study by Ernst et al,32 80 patients underwent open aortic repair. A greater number of positive cultures was found in those patients with ruptured aneurysms compared with asymptomatic and symptomatic aneurysms (38% versus 9% and 13%, respectively). More recent research has further highlighted the potential significance of bacterial infection in the etiology of degenerative aortic aneurysms33 (Figs. 142-1 and 142-2).
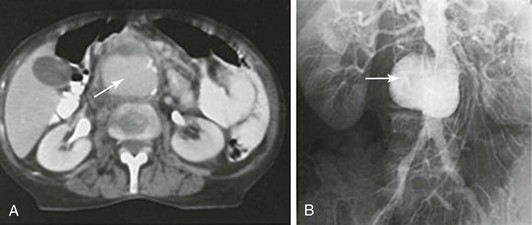
Figure 142-1 Diagnostic radiology studies of a patient with salmonella infection of a preexisting small atherosclerotic aneurysm. A, Contrast-enhanced CT scan showing a saccular aneurysm with calcification (arrow). B, Transfemoral aortogram showing a saccular atherosclerotic infrarenal aneurysm (arrow).
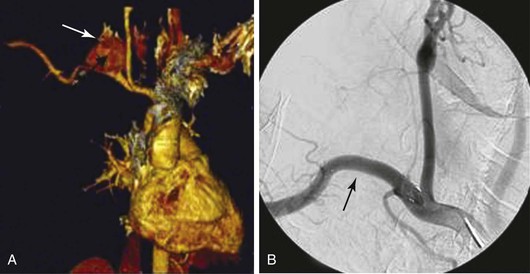
Figure 142-2 A, Three-dimensional reconstruction CTA image in a patient with a polymicrobial posttraumatic false aneurysm of the right subclavian artery caused by repeated percutaneous cervical injection of illegal narcotics (arrow). B, Treatment with a covered stent-graft for control of hemorrhage (arrow). Adjuvant therapy included open débridement and irrigation along with intravenous antimicrobial therapy.
Infected Aneurysms from Cardiac Source
Although infected aneurysms caused by endocarditis were the first described by Osler,1 currently less than 10% of infected aneurysms originate in this manner.15,34,35 Septic emboli of cardiac origin may lodge in the lumen or occlude the vasa vasorum of the arterial wall, leading to ischemia and infection of the arterial wall. Once the artery is infected, rapid, focal, and progressive deterioration occurs, which results in the characteristic saccular or multiloculated appearance of these aneurysms. This process often leads to a locally contained rupture and formation of a false aneurysm.14,21 When infected aneurysms are associated with cardiac emboli, they can be multifocal and are most commonly seen in the aorta, intracranial circulation, and splanchnic and femoral arteries, typically at arterial bifurcations.1,2,36
Bacteriology
The predominant microorganisms found in infected aneurysms have evolved with the advent of antibiotics and depend somewhat on the type and etiology of the aneurysm, as well as where the patient resides geographically and whether they are in an immunosuppressed state. The bacteriologic spectrum that is seen in these patients is extensive, and may be broader than was once thought.37 As a general rule, the following rates apply in current practice. Staphylococcus species are the most common organisms, and account for 28% to 71% of cases. Salmonella species are the second most common, and have been reported in 15% to 24% of patients, with Streptococcus species reported in less than 10% of the cases in the postantibiotic era,8,13 having been a much more common cause before the advent of antibiotics.38
Overall, blood cultures are positive in 50% to 85% of infected aneurysm patients,7,15,39 and organisms have been isolated from aneurysmal tissue in up to 76% of patients with a suspected infected aneurysm.15,40 Despite the constant evolution of bacteriologic patterns, organisms like Staphylococcus and Salmonella species remain the most common, and are known to have the greatest affinity for the arterial wall.41–43 As emerging technologies based on endoluminal technologies gain popularity, infection in an existing aneurysm and microbial arteritis may be associated with postimplantation infection.44,45
Specific Bacteria
Although less common, gram-negative infections are more virulent than gram-positive infections from the standpoint of aneurysm rupture (84% versus 10%) and patient mortality (84% versus 50%), respectively.19 The reason for this increased virulence is postulated to involve the ability of gram-negative organisms like Pseudomonas aerugenosa to release alkaline proteinase along with a variety of elastases that cause vascular wall necrosis.46 Furthermore, gram-negative organisms are also commonly implicated in graft disruption and arterial stump hemorrhage after reconstruction, and the presence of these bacteria should be taken into careful consideration when planning repair.
Methicillin-resistant Staphylococcus aureus (MRSA), which causes soft tissue infections and infected aneurysms, is becoming an important public health problem. New strains of S. aureus displaying unique combinations of resistant traits have been associated with high morbidity and mortality, and several series report MRSA as the predominant organism in infected aneurysms.47–49
The diseased aorta appears to be particularly vulnerable to salmonella, and this pathogen is frequently found in infected preexisting aneurysms and in the infected atherosclerotic nonaneurysmal aorta. Specifically, S. choleraesuis and S. enteritidis account for more than half of reported cases of salmonella aortitis.34,50
Other organisms uncommonly cause infected aneurysms. Fungal aneurysms have been reported in patients with diabetes mellitus, immune suppression, and those with a history of systemic fungal disease.4,51,52 Reported fungal pathogens include Candida,53 Cryptococcus,54 Aspergillus,55 and Pseudallescheria boydii,56 among others.
Treponema pallidum and mycobacterium species have also been implicated in infected aneurysms.32,57–59 Interestingly, syphilis (T. pallidum) once caused up to 50% of infected aneurysms, but has been much less common since the advent of antibiotics. Finally, tuberculosis is a rare cause of infected aneurysms and is generally described to be secondary to erosion of the periaortic lymph nodes into the aortic wall.60 More recently, the use of Bacillus Calmette-Guerin (attenuated bovine tuberculosis bacillus) as an intravesical treatment for superficial bladder cancer has led to vascular infections at distant sites as well, including the infrarenal aorta and popliteal artery.61,62
Diagnosis
Clinical Findings
The presentation of an infected aneurysm will depend on the anatomic location, the virulence of the organisms, and the duration of the infection. Specific discussion of symptoms and diagnosis of each anatomic location is described later in this chapter. General findings include fever, chills, leukocytosis, back pain or abdominal pain for aortic aneurysms, and distal embolization, sometimes with pulsatile masses and overlying cellulitis for peripheral aneurysms.16,19,63–65 If untreated, infected aneurysms typically lead to sepsis and can certainly rupture, causing life-threatening hemorrhage.
Although some patients present as overtly septic, in some cases diagnosis can be difficult, with nonspecific symptoms such as malaise and subjective fevers. For those aneurysms in more superficial locations, such as the femoral, popliteal, carotid, and upper extremity arteries, local signs of infection can be identified, usually as a pulsatile mass with or without associated cellulitis.14,16,22,66 Patient survival depends on prompt diagnosis and definitive management,19,64 and a high index of suspicion should be maintained in certain clinical scenarios. Infected aneurysms should be suspected when the aneurysm is found in conjunction with positive blood or tissue cultures, erosion of vertebrae adjacent to an aortic aneurysm, rapid aneurysm growth, an uncalcified aneurysm in an older patient, or initial manifestation of an aneurysm after bacterial sepsis, especially in immunocompromised patients.19,21,67,68
Laboratory Studies
Leukocytosis and elevated erythrocyte sedimentation rates are common, but nonspecific findings often present in infected aneurysms.69 If patients are treated with antibiotics before obtaining the definitive diagnosis, the sensitivity of these findings is, of course, limited.52 Positive blood cultures without other obvious sources in patients with a degenerative aneurysm should raise diagnostic suspicion, and treatment should be initiated when the suspicion is high. Negative blood cultures or the lack of the previously listed findings are not sufficiently sensitive to rule out the diagnosis of an infected aneurysm.12
When infection is suspected, intraoperative cultures should always be obtained, with sampling of the arterial wall and thrombus for aerobic and anaerobic bacteria, as well as fungal cultures to help direct postoperative antibiotic therapy. Intraoperative gram stain can be important to help determine the conduit and the method of revascularization, because certain organisms have been implicated in graft degeneration and catastrophic hemorrhage when in-situ reconstruction is chosen.70 Importantly, a negative gram stain does not rule out the possibility of infection,71 and final culture results may be misleading in patients treated preoperatively with antibiotics.
Imaging
Radiologic studies are useful for obtaining the diagnosis and planning of surgical reconstruction. Ultrasonography and arterial duplex can been helpful in the initial diagnosis of femoral, carotid, lower and upper extremity aneurysms, but is not specific and cannot confirm the diagnosis.16 Currently, computed tomographic angiography (CTA) is readily available and has become the standard test for infected aneurysms. CTA findings consistent with an infected aneurysm may include lack of calcification, saccular, multiloculated or eccentric aneurysms, soft tissue inflammation or surrounding mass, air within the aneurysm or surrounding tissue, periarterial fluid collections, pseudoaneurysm formation, or contained rupture. Short-term serial CT scans may be valuable in suspected cases when the initial CT findings are nondiagnostic. Short-term studies that reveal rapidly enlarging or rapidly evolving aneurysms are highly suggestive of infection (Fig. 142-3).72–75
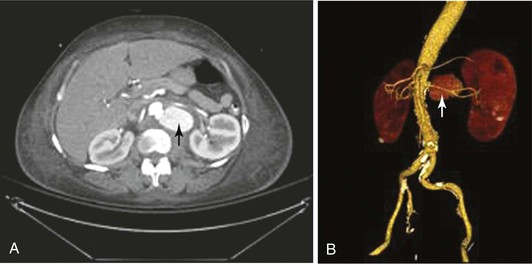
Figure 142-3 Axial and reconstructed CTA images of a patient with Salmonella microbial aortitis and an aneurysm. A, CTA showing contained rupture of the infected aneurysm (arrow). B, Three-dimensional reformatting showing a saccular eccentric infected aneurysm of the juxtarenal aorta (arrow).
Positron emission tomography (PET) alone or in combination with CT has also been used effectively for the detection of arterial infections, given the often avid uptake of the radionuclide tracer by infected tissues. A number of authors have reported using these imaging methods for infected aneurysms in various anatomic locations, but nuclear imaging has certainly not received widespread acceptance for this application.76–78
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) may prove helpful for screening certain anatomic sites or when CT contrast media are contraindicated; both modalities are highly sensitive for inflammation.79,80
Indium 111–labeled white blood cell scanning (“tagged white blood cell scan”) has been used in some series to identify prosthetic graft infections, but its use has not always been accurate in primary infections.81,82 Although not sensitive or specific, these studies may be particularly helpful when other imaging studies are equivocal. Finally, Australian investigators have reported the utility of bone and leukocyte scintigraphy to show the extent of adjacent soft tissue and lumbar osteomyelitis in a case of ruptured infected abdominal aortic aneurysm,83 but this has not been widely adopted as an imaging method in those situations as of yet.
Antibiotic Treatment
Antibiotic therapy has an important role in the treatment of infected aneurysms in every location; these should be initiated as soon as possible and should be continued after surgical treatment. Broad-spectrum antibiotics should be the initial choice, and then narrowed to culture- and sensitivity-directed therapy once this information is available. Because of the importance of directed antibiotic therapy, the importance of blood culture before initiating antibiotics along with tissue culture at repair cannot be overemphasized. The duration of the antibiotic course is not well established and can vary from weeks to lifelong courses, especially in cases of highly virulent organisms or multidrug resistant pathogens. A minimum of 6 weeks of intravenous antibiotic therapy is recommended in most series.21,84 Especially in locations where recurrent infection is often lethal, such as aortic infection, most physicians have erred toward longer, even lifelong, suppressive antibiotic treatment in these high-risk patients.
Operative Treatment
The operative treatment of infected aneurysms depends somewhat on anatomic location, and a detailed outline of treatment options by anatomic location is discussed in the following sections. The following general principles apply to the treatment of all infected aneurysms.
Control of hemorrhage through proximal and distal control should be obtained, and intraoperative gram-stain and tissue samples should be sent for aerobic, anaerobic, and fungal culture. Operative control of sepsis should be performed with resection, including the aneurysm and all surrounding necrotic or infected tissues.
Pre- and postoperative antibiotic therapy should be broad spectrum, and include vancomycin and an agent that covers gram-negative organisms (especially salmonella) until organism-specific antibiotic therapy can be instituted. The duration of therapy varies and is at the discretion of the surgeon based on the location of the aneurysm, virulence of the organism, and any drug resistance. A minimum of 6 weeks duration of intravenous antibiotics is recommended, with culture-directed suppressive lifelong oral antibiotics, when considered appropriate by the clinician.
Revascularization through noninfected tissue planes should be used with extra-anatomic bypass, or an in-situ reconstruction may be performed with autogenous conduit (most desirable), cryopreserved allografts, or prosthetic grafts (least desirable). Selection and need for reconstruction are guided by multiple factors, including the surgeon’s experience, the patient’s surgical risk and comorbidities, the anatomic location of the aneurysm, and the availability of autogenous conduit. Ideally, the graft should be covered by well vascularized tissue, and if necessary, omental or muscle flaps should be used to augment coverage.
Aorta
Infected aortic aneurysms have been described in all segments from the aortic root to the aortic bifurcation. Oderich et al7 reported the following distribution of infected aneurysms: infrarenal aorta (40%), distal thoracic aorta (16%), thoracoabdominal segment (16%), paravisceral aorta (13%), juxtarenal aorta (11%), and pararenal aorta (4%) (Fig. 142-4).
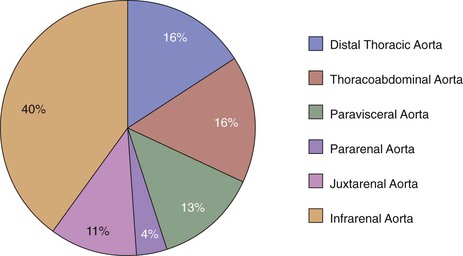
Figure 142-4 Distribution of infected aortic aneurysms in a report of 43 consecutive patients from the Mayo Clinic. (From Oderich GS, et al: Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg 34:900-908, 2001.)
The diagnosis of infected aortic aneurysms is based on clinical findings and imaging features. Most patients are asymptomatic; however, the presence of fever, chest pain, and dyspnea (for thoracic involvement), back pain, and leukocytosis should raise suspicion. Blood cultures have been reported to be positive in approximately 75% of cases.7
CTA has become the standard test for diagnosis of infected aortic aneurysms. The presence of localized saccular aneurysm or pseudoaneurysm is pathognomonic; periaortic collections, intramural air or air collection around the vessel, soft tissue infiltration, and rapid aneurysm expansion are other common radiographic findings. Despite the significant morbidity and mortality associated with infected aortic aneurysms, outcomes have improved in the last 15 years, with the outcome after presentation being mostly dependent on the rupture status of the aneurysm and the time to intervention.85
Thoracic Aorta
Infected thoracic aortic aneurysms are highly lethal, with an associated mortality reported to be 30% to 50%. The most common organisms are Salmonella and Staphylococcus species, and those patients with salmonella infections have the worst clinical outcome. Symptoms are usually nonspecific, and the most common clinical presentation is rupture. In one of the largest experiences reported in the literature, which included 24 patients, of whom 19 underwent operation and 6 had aneurysms in the thoracic aorta, Hsu et al86 reported that 89% of their primary infected aneurysm patients had a pseudoaneurysm or frank rupture noted at operation.
Treatment
Surgical excision of the infected segment, wide debridement, and long term-intravenous antibiotics remain the definitive treatment for infected thoracic aneurysms. In-situ reconstruction is the most common approach, usually with a homograft or antibiotic treated prosthetic graft. Repair is done via left thoracotomy for descending aortic aneurysms and the left thoraco-retroperitoneal approach for those aneurysms with thoracoabdominal involvement. As with degenerative aneurysm repair, special attention must be given to spinal cord perfusion protection maneuvers during this repair.87
Complications
Surgical complications are similar to those related to noninfected thoracic aneurysm repair, including cardiovascular, cerebrovascular, pulmonary, spinal cord ischemia, and renal failure. Crawford type II, advanced age, and contained rupture are associated with higher 30-day mortality.88 Mortality of 85% has been reported in those patients who were managed with antibiotic therapy only, with in-hospital rupture in two thirds of the patients.89
Endovascular Treatment
More recently, treatment using endovascular stent grafts combined with antibiotic therapy has been described as an alternative to conventional thoracotomy in managing infected aneurysms of the thoracic aorta. This may be an especially attractive option in patients who are poor surgical candidates. Unfortunately, the lack of debridement is a risk for continued infection, and durability of the graft and long-term outcomes related to the infection are yet to be determined.90–92 Further discussion of endovascular therapies are outlined later in this chapter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree