Placental oxygenation is a major characteristic of fetal circulation. Additionally, in fetal circulation, blood flow through the right and left sides of the heart occurs in parallel, and therefore cardiac output is actually combined ventricular output (CVO).1 This is possible as a result of shunts present in fetal circulation.2 Three points of shunting during fetal circulation are at the ductus venosus, foramen ovale, and ductus arteriosus. The oxygenated blood from the umbilical vein passes through the ductus venosus to the foramen ovale.1 The foramen ovale allows right-to-left blood flow through the atria, bypassing the lungs.1,2,3 Left ventricular output (LVO) enters the ascending aorta and flows to the brain and upper body. The ductus arteriosus similarly allows the lungs to be bypassed, as most of the right ventricular output (RVO) flows to the descending aorta and from there to the lower body.1,4 RVO is larger than LVO (59% and 41%, respectively).5 Fetal blood flow to the lungs from the right ventricle is low because of high pulmonary vascular resistance during fetal circulation. Only 10% to 12% of CVO goes to the lungs,5–7 and its function is to nourish the developing lung tissue rather than to provide gas exchange. Adult-like circulation occurs at or shortly after birth, with separation from the placenta and ventilation of the lungs resulting in closure of the ductus venosus, foramen ovale, and ductus arteriosus.1,8 This process, referred to as transitional circulation, occurs early in neonatal life and increases the efficiency of oxygen uptake and transport.2,4,9 The initiation of breathing and removal of lung fluid increase pulmonary blood flow. Whereas fetal circulation is characterized by high pulmonary vascular resistance and low systemic resistance, separation from the placenta causes a rise in systemic resistance and a decrease in pulmonary vascular resistance. As this shift in relative pulmonary and systemic resistances occurs, the shunts close and the ventricles shift from working in parallel to working in series.9,10 The increase in left atrial pressure that occurs during transitional circulation results in apposition of the valve of foramen ovale against the interatrial septum, functionally closing this site of fetal circulatory shunting.10,11 The anatomical closure occurs several weeks to several months later.11 Functional closure or constriction of the ductus arteriosus occurs within the first 24 to 48 hours after birth in response to increased arterial oxygen saturation. Anatomical closure of ductus arteriosus occurs by 2 to 3 weeks in most term neonates.4 The responsiveness of the ductal smooth muscle to arterial oxygen tension and to endogenous prostaglandins is affected by gestational age at birth.4,12 Structural and functional characteristics of pulmonary development in infants and children are significant because they may contribute to specific aspects of respiratory vulnerability.10 The pulmonary anatomy of the term infant is markedly different from the adult but also different from the child. An infant’s airways are narrower from the nares to the terminal bronchioles. This presents a point of pulmonary vulnerability because a smaller-diameter airway is more easily obstructed by mucus, edema, foreign objects, and enlarged lymphatic tissue. The infant also has a higher larynx.10 Although this position of the larynx enables the newborn to breathe and swallow simultaneously, it may also contribute to predominant patterns of infant nasal breathing, which may result in increased work to breathe during any compromise of the nasal airway. Although the process of transitional circulation allows more efficient oxygen transport in the neonate than in the fetus, gas exchange in the newborn is still somewhat inefficient because of immature alveolar structure and function, with significantly lower number of alveoli present in the lungs of a newborn as compared to an adult.3 The surface area for gas exchange in a newborn is one-twentieth that of an adult,13 and the diffusion distance across the alveoli-capillary membrane is increased because of thick alveolar walls.14 Functional characteristics of infant pulmonary development are also significant in terms of understanding possible contributions to pulmonary distress in infants. The diaphragm of a newborn has fewer type I (fatigue-resistant) muscle fibers (20% compared with 50% in an adult).10 This difference predisposes an infant to earlier diaphragmatic fatigue when stressed. A biomechanical difference between the infant and child is the circular and horizontal alignment of the ribs and the concomitant horizontal angle at which the diaphragm inserts on the ribs during newborn and early infant chest development.8,10,15 This, along with the more cartilaginous nature of the ribcage, results in less efficient chest wall mechanics, leading to increased work of breathing (see Chapter 39 for a more complete discussion of chest development). Two residual structural differences exist beyond the newborn period and have potential implications for pulmonary vulnerability in the child. First, the somewhat horizontal angulation of the ribs persists until approximately 10 years of age and results in less efficient chest wall mechanics.16 Second, although lymphatic tissues, including tonsils and adenoids, grow in proportion to skeletal growth from 1 to 11 years of age,17 they may be a potential source of upper airway obstruction in some children. As infants and children grow and develop, however, most structural and functional disadvantages disappear. An aspect of growth and development that can be protective for infants and younger children is alveolar multiplication. This begins in the first year of life and continues until approximately 8 years of age.3,8 All of the cardiovascular and pulmonary structural and functional characteristics of neonates that have been previously discussed apply to the premature infant. Additionally, there are significant gestational aspects of cardiovascular and pulmonary vulnerability that are more pronounced and create more problems in premature infants (Table 37-1). Table 37-1 Factors Contributing to Cardiovascular and Pulmonary Dysfunction in the Premature Infant Modified from Crane LD: Physical therapy for the neonate with respiratory disease. In Irwin S, Tecklin JS, editors: Cardiopulmonary physical therapy, St Louis, 1995, Mosby. Recall that the transitional circulation of a term newborn includes a decrease in pulmonary vascular resistance over the first day. In preterm infants, however, lung immaturity and abnormal surfactant function18 may result in retained high pulmonary resistance (poor lung compliance), hypoperfusion, and respiratory distress syndrome.3,19,20 Persistent pulmonary hypertension will reinforce persistent right-to-left shunting through the ductus arteriosus.21 Respiratory distress syndrome (RDS) has been identified as the best predictor of prolonged patency of ductus arteriosus.22 As the respiratory distress of the premature infant improves with neonatal intensive care, cardiovascular and pulmonary vulnerabilities can then occur in the opposite circulatory direction. Given the gestationally related responsiveness of ductus arteriosus to oxygen, some preterm infants retain patency of the ductus even when pulmonary vascular resistance falls.23 The result is left-to-right shunting that leads to excessive pulmonary blood flow and the need for supplemental oxygen and mechanical ventilation,24 which increases the risk for bronchopulmonary dysplasia.25,26 Therefore the immature status of cardiovascular and pulmonary anatomy in preterm infants predisposes them to hypoxia under any conditions that require increased oxygen.14,18 Cardiovascular and pulmonary pathophysiology and the stressors inherent to medical care often present challenges beyond the adaptive capacities of these fragile little systems. Patent ductus arteriosus, endocardial cushion defects, and tetralogy of Fallot are the cardiac defects discussed within the limits of this chapter. Additionally, Table 37-2 provides a summary of common developmental diagnoses and their associated cardiac defects and conditions. Table 37-2 Common Pediatric Diagnoses and Associated Heart Defects and Conditions Patent ductus arteriosus (PDA), already introduced as a cardiopulmonary complication in preterm infants, is the most common heart defect during the neonatal period.24,36 In term newborns, however, PDA incidence is 1 in 2000 births (5% to 10% of infants with congenital heart disease).4 Gestational age, the presence of lung disease, the size of the ductus, and the direction of the shunt mediate the clinical features of PDA.24,36,43 The preterm infant with very low birth weight will have the most extreme clinical picture. An infant or child with a large ductus will also have an obvious clinical presentation. Tachycardia, an ejection systolic murmur, bounding peripheral pulses, increased respiratory distress, and poor feeding or poor weight gain are the classical signs of PDA.4,23,36 In the term infant, PDA may be more clinically subtle, especially when the ductus is small.4 Depending on the extent of the PDA, medical management includes nonsurgical (intravenous) use of indomethacin or direct, minimally invasive surgical closure.24,36,44–46 Endocardial cushion defects (ECD) or atrioventricular septal defects (AVSD) represent a spectrum of defects characterized by malformation of the atrial septum, the mitral and tricuspid valves, and/or the ventricular septum.47 Combinations of these defects are categorized as complete, transitional/intermediate, and partial, depending on degree of ventricular septal deficiency.48–50 In the complete form, all of the structures are deficient. In the partial form of ECD, only an atrial septal defect with a cleft mitral valve is present.47–49 There is marked variation in the underlying anatomy of this class of cardiac defects, such that clinical features are equally varied and difficult to summarize inclusively. In neonates with a complete defect, heart failure may manifest in infancy. Children with milder forms of this defect, however, may not be symptomatic until much later in life, and sometimes, not until adulthood.49 Additionally, it is common for ECD to be associated with other cardiac defects.47 Although the total incidence of endocardial cushion defects in infancy is 1% to 4%, the incidence in infants and children with Down syndrome is 40% to 50%.51,52 A complete AVSD is associated with this genetic disorder.50 Operative management of infants and children with ECD depends on the morphology of the defect, the degree of pulmonary hypertension, and the extent of mitral valve regurgitation.48 For infants with complete AVSD, early surgical repairs are indicated.52,53,50 Tetralogy of Fallot (TOF) is named for its tetrad of defects: ventricular septal defect, right ventricular outflow obstruction, right ventricular hypertrophy, and aortic override.54,55 A neonate with TOF will have symptoms dependent on the extent of right ventricular tract obstruction, which results in decreased pulmonary blood flow and the presence of right-to-left shunting.54 The classical picture is one of cyanosis, especially with crying or agitation.47,55 Although most infants have sufficient pulmonary blood flow at birth, the cyanosis usually increases within a few weeks or months after that.54 In neonates, initial medical management may include treatment of hypoxemia by pharmacologically maintaining patency or reopening the ductus arteriosus for additional pulmonary blood flow.55,56 The elimination of conditions that produce hypoxemia may also include pharmacological agents to increase systemic vascular resistance and decrease myocardial contractility.54 Surgical repairs are generally performed between 3 and 6 months of age.54,55 The most common respiratory disorder in infants born prematurely is respiratory distress syndrome (RDS), or hyaline membrane disease. The higher incidence of RDS has been linked to lower birth weights, and this condition has been described as the primary cause of mortality and morbidity in this population.3,18,20 A correlation between RDS and increased morbidity, including apnea, bradycardia, and pneumonia, has been reported.57 RDS results from lung immaturity, insufficient number of alveoli, and an inadequate amount of surfactant.3,7,20 Surfactant decreases alveolar surface tension and prevents alveoli collapse during expiration. The surfactant deficiency results in alveolar collapse and increased work of breathing.20 Antenatal corticosteroids20,58 and surfactant replacement are standard care for prematurely born neonates to reduce symptoms and sequelae of RDS.3,59,60 Respiratory failure is common in these infants and necessitates oxygen therapy and assisted ventilation, which, in turn, may lead to further respiratory problems, lung injury, and bronchopulmonary dysplasia.3,61,62 Chest physical therapy (CPT) has been commonly advocated for airway clearance and to decrease postintubation atelectasis in infants with RDS.63–66 However, concerns have been raised about possible brain damage associated with CPT in infants with extreme prematurity and very low birth weight.65,67 Evidence supporting the application of this intervention with preterm infants has been insufficient and, at times, contradictory.66,67,68 The results of a Cochrane review of effects of percussion and vibration on the pulmonary system in mechanically ventilated infants were also inconclusive but showed no increase in risk for intraventricular hemorrhage associated with these CPT techniques.66 As more preterm newborns survive neonatal respiratory distress, the prevalence of chronic lung disease, or bronchopulmonary dysplasia (BPD), has increased.62,69 Although in the past the etiology of BPD was strongly linked to high-pressure positive pressure ventilation and oxygen therapy in the treatment of respiratory distress during the neonatal period,18,70 this condition is now also seen in infants who initially may need only low-pressure ventilation and minimal oxygen supplementation but whose respiratory function deteriorates over time, with an increased demand for oxygen and ventilatory support seen later, after prolonged mechanical ventilation.62 Oxygen toxicity, combined with damage to the alveoli caused by mechanical ventilation, leads to inflammation, pulmonary edema, and fibrosis.61,62,71 The risk for BPD is highest in infants born at 32 weeks of gestation or earlier, with a birth weight ≤1200 grams, and in those who have more severe RDS.3,62,71 Table 37-3 displays the definition and diagnostic criteria for BPD.71 Table 37-3 Definition of Bronchopulmonary Dysplasia: Diagnostic Criteria *A physiologic test confirming that the oxygen requirement at the assessment time point remains to be defined. This assessment may include a pulse oximetry saturation range. BPD usually develops in neonates being treated with oxygen and positive pressure ventilation for respiratory failure, most commonly respiratory distress syndrome. Persistence of clinical features of respiratory disease (tachypnea, retractions, rales) are considered common to the broad description of BPD and have not been included in the diagnostic criteria describing the severity of BPD. Infants treated with oxygen >21% and/or positive pressure for nonrespiratory disease (e.g., central apnea or diaphragmatic paralysis) do not have BPD unless they also develop parenchymal lung disease and exhibit clinical features of respiratory distress. A day of treatment with oxygen >21% means that the infant received oxygen >21% for more than 12 h on that day. Treatment with oxygen >21% and/or positive pressure at 36 wk PMA, or at 56 d postnatal age or discharge, should not reflect an “acute” event, but should rather reflect the infant’s usual daily therapy for several days preceding and following 36 wk PMA, 56 d postnatal age, or discharge. Reproduced with permission from Jobe AH, Banclari E: Bronchopulmonary dysplasia, Am J Respir Crit Care Med 163:1723-1729, 2001. Medical management of BPD is primarily supportive. Long-term oxygen therapy is often necessary for infants who exhibit persistent severe hypoxemia. The target oxygen saturation levels administered via several different strategies is 90% to 95%.72 The most promising reported ventilatory strategies include avoiding intubation whenever possible, administering surfactant early if the infant has to be intubated, and using noninvasive ventilation, such as nasal continuous positive airway pressure (CPAP) or synchronized nasal intermittent positive pressure ventilation (SNIPPV).72,73 Such sequelae of BPD as congestive heart failure, cor pulmonale, and pulmonary edema warrant diuretic therapy for these infants.18 Many infants with BPD require rehospitalization before 1 year of age, with respiratory symptoms persisting beyond infancy and into childhood, adolescence, and even adulthood. Decreased lung function in these children3 has been linked to the duration of oxygen supplementation.72 Because BPD survivors demonstrate decreased growth and increased incidence of neurodevelopmental sequelae, including cerebral palsy, impairments in gross and fine motor skills, cognition and language development, many of them require developmental interventions, such as physical, occupational, and speech therapies.18,72,74 Evidence related to CPT application for airway clearance with infants born preterm has been discussed earlier in this chapter. Transient tachypnea of the newborn (TTNB) is another neonatal problem considered in the differential diagnosis of RDS. It is associated with delayed clearance of amniotic fluid from the lungs, and is the most common cause of respiratory distress in neonates.47,75 It is a self-limiting condition that usually resolves spontaneously, with symptoms lasting from 2 hours to 2 days. Risk factors for TTNB include cesarean section delivery, macrosomia (birth weight of greater than 4000 grams), male sex, and maternal asthma or diabetes.75 Meconium is the content of fetal and newborn bowels. Meconium aspiration syndrome (MAS) is respiratory distress in a full-term or post-term neonate born through meconium-stained amniotic fluid (MSAF). This diagnosis is made in absence of any other explanation.76,77 Meconium aspiration may occur with the first postnatal breaths or with gasping in utero just before delivery.76,78,79 Although in the past, it was suggested that MAS could be prevented if the upper and lower airways were suctioned immediately after birth,80 more recent research showed no benefit from routine intrapartum oronasopharyngeal suctioning and yielded varied recommendations regarding endotracheal intubation and lower airway suctioning performed immediately after delivery.77,81,82 Generally, intubation is recommended for “depressed” but not “vigorous” infants born through MSAF. A systems approach to prevention of MAS has been proposed, including careful monitoring of the delivery by a well-trained interdisciplinary rapid response team and an evidence-based decision-making process regarding the need for endotracheal intubation and suctioning.81 Aspiration of meconium may result in serious and devastating pathophysiology. Most commonly, the meconium will partially or completely block the peripheral airways.76–79 Atelectasis is the classical finding, but with partial obstruction, hyperexpanded areas will also be observed as the result of air trapping in distal, small airways after inspiration. Common complications of MAS include tension pneumothorax, persistent pulmonary hypertension, bronchiolitis, and meconium-induced chemical pneumonitis.76–79 Additionally, long-term pulmonary sequelae, such as BPD, and neurological and neurodevelopmental disorders, such as seizures, cerebral palsy and global delays, have been reported in these infants.76,83,84 Medical management of MAS includes respiratory support via supplemental oxygen and, if necessary, mechanical ventilation; extracorporeal membrane oxygenation (ECMO); and surfactant administration.82 CPT has been advocated for infants with MAS,85,86 but more research is needed to confirm its benefits for this patient population. In infants with perinatally acquired HIV, Pneumocystis jiroveci pneumonia (PCP) frequently occurs within the first 12 months of life, with the peak incidence reported at 3-6 months of age. Although in the United States there has been a decline in the rate of PCP infection, it remains the leading cause of death in children with HIV/AIDS in Africa.90 The clinical presentation of PCP includes failure to thrive, cough, dyspnea, tachypnea, fever, hypoxemia, and chest radiograph evidence of diffuse parenchymal infiltrates of reticulogranular quality.90,91 PCP is most definitively diagnosed after bronchoalveolar lavage studies. Chemoprophylaxis is recommended to prevent the first episode of this disease. Treatment includes antibiotic therapy, antiretroviral therapy, nutritional support, short courses of corticosteroids, and surfactant administration in severe cases.90 Although asthma is described in detail in Chapter 5, a discussion of pediatric pulmonary problems would not be complete without mentioning this common cause of childhood lung disease. Currently, approximately 22% of school children in the United States have asthma, with this disease prevalence ranging from 15.5% to 28.3% among different states.92 Childhood asthma can begin at any age, and its clinical etiology and clinical course are variable. Children with early medical histories that include very low birth weight, bronchopulmonary dysplasia, and respiratory syncytial virus (RSV) infection may be at increased risk for developing asthma.93–95 Common triggers for asthma symptoms and exacerbations are allergens, respiratory viral infections, tobacco smoke, air pollutants and irritants, extreme temperatures and high humidity, excessive weight gain and low physical fitness, stress, and exercise leading to bronchospasm.96 Medical management usually includes avoidance of known precipitants, bronchodilator drugs, and corticosteroids (in chronic, severe cases). Other measures include immunotherapy and child, parent, and health-care provider education, as well as monitored exercise to maintain healthy weight and fitness.96,97 Asthma is underdiagnosed and undertracked among minority groups and children from families with low socioeconomic status (SES).98 Some studies suggest that detection and treatment in schools may be part of the solution.98,99 Similarly, education regarding asthma management of children from minority or low SES families may be effectively implemented in schools by positioning the school nurse as the case manager.98–101 In a school district that does not have a full-time nurse in each school, the physical therapist may need to track and be aware of asthma in the child before he or she engages in physical exertion. Cystic fibrosis (CF) is a complex, autosomal-recessive disorder that occurs at a frequency of one in 3500 live births.102 A defect in a single gene, the CF transmembrane conductance regulator (CFTR), leads to pathological changes in exocrine glands, including airways, sweat glands, pancreas, biliary tract, and gut.103,104 The Cystic Fibrosis Foundation Consensus Statement on the Diagnosis of CF102 includes recommendations for newborn screening (NBS) to identify infants at risk for CF and for the sweat chloride test as the primary method used to confirm the diagnosis. The diagnostic pathway for screened newborn babies differs from that used for patients who present with clinical symptoms of CF. Positive results of NBS warrant a sweat chloride test, which, if also positive, is followed by genetic testing. In patients with signs and symptoms of CF and a family history of the disease, a sweat chloride test is performed, followed by a clinical evaluation, genetic testing, pancreatic function tests, and tests for specific pathogens, such as Staphylococcus aureus or Pseudomonas aeruginosa, present in the respiratory tract. Clinical symptoms of CF are chronic productive cough, respiratory infections with CF pathogens, abnormal chest radiographs, airway obstruction, nasal polyps, and clubbing of the digits. Other symptoms include gastrointestinal and nutritional problems, systemic loss of salt, and male genital abnormality.102 The disease progression is one of chronic lung infection that results in fibrosis and bronchiectasis that lead to respiratory insufficiency, the primary factor in morbidity by the second or third decade of life.103,104 Chronic nocturnal hypoxia becomes a stimulus for the development of pulmonary hypertension and right ventricular failure and is associated with poor prognosis.105 The chronic pulmonary disease in CF is related to increased secretion of abnormally viscous mucus, impaired mucociliary transport, airway obstruction, bronchiectasis, overinflation, and infection.102 Early detection and early treatment are key to the management of CF. The major marker of pulmonary disease in this population is forced expiratory volume in 1 second (FEV1).106 However, one study that used high resolution computed tomography (HRCT) to examine the lungs of children 6 through 10 years of age who had no abnormality of FEV1 revealed air trapping and bronchiectasis.107 Similarly, when FEV0.5 was used as a marker, airway function was noted to be diminished soon after diagnosis in infants with CF.108 These results support the importance of early intervention in children for whom detection occurs during infancy.106 Further development of genetic diagnostics and gene therapy may enhance early identification and address effective, if not preventive, early treatment.109,110 Medical management includes early nutritional support, treatment of pancreatic dysfunction, judicious use of antibiotics, and preventive infection control measures.111 The early institution of prophylactic pulmonary physical therapy, such as airway clearance techniques, helps to control or decrease the effects of bronchiolar and bronchial obstruction. Involvement of the child and family in pulmonary care is particularly important. Family understanding of the nature of the disease and the purpose of each therapeutic measure promotes successful intervention. An individualized home program of CPT should be established, taking into consideration the child’s ongoing pulmonary needs and the family’s unique contributions and constraints. Bronchial drainage is an aspect of conventional treatment for CF with several options that allow both efficacy and patient independence.112 Airway clearance techniques (ACT) are described in detail in Chapter 21, but alternatives to traditional percussion and postural drainage warrant mention within the context of CPT for children with CF. Specifically, the forced-expiration technique (FET) as part of the active cycle of breathing (ACB) technique, the use of a positive expiratory pressure (PEP) mask, autogenic drainage (AD), the use of the flutter device, and the application of high-frequency chest compression (HFCC) through an inflatable vibrating vest have been shown to be effective in assisting sputum expectoration, often in greater amounts and in less time per treatment compared with other interventions.113–119 The efficacy of PEP in this population remains equivocal.120,121 Although postural drainage, percussion, and vibration are the treatment of choice for infants and children who are unable to be instructed in techniques that use specific patterns of voluntary breathing, other techniques are available for children who can follow breathing instructions and who can perform a reliable pulmonary function test. Children as young as 2 to 3 years of age can be taught to “huff” as part of FET;113 the PEP mask has been used with children as young as three years;115 and AD can reportedly be taught to children at 4 to 6 years of age.116 The Cystic Fibrosis Foundation CF Pulmonary Guidelines119 recommend ACT for patients with CF to maintain lung function, clear sputum, and improve their quality of life. Because none of these techniques have been shown to be superior to others, it is recommended to base their prescription on the child’s age, specific technique preference, and incidence of adverse events associated with the use of ACT.119 In patients diagnosed with CF, an airway clearance program should be initiated in early infancy.111 The benefits of each technique should be carefully considered relative to each child’s cognitive, respiratory, and motor planning abilities when choosing or modifying the airway clearance program, and the child’s response to any technique must be carefully monitored. The head-down position should not be used for infants and toddlers younger than 2 years of age because it may exacerbate gastroesophageal reflux and affect oxygen saturation in infants with CF who have gastroesophageal reflux disease.111,119,122 Ventilatory muscle training is an important aspect of pulmonary treatment in older children with CF. Studies have demonstrated that training to improve the endurance of ventilatory muscles decreases dyspnea and increases general exercise tolerance in patients with CF.123,124 The role of general exercise in the cardiovascular and pulmonary management of children with CF is discussed within the context of pulmonary rehabilitation later in this chapter. It is important to mention that the use of aerobic exercise for patients with CF is recommended for general health and fitness benefits, as well as a component of the airway clearance program.104,119 Once an infant or child develops respiratory failure, intubation and mechanical ventilation are usually required.3,125 The goals of medical management are then to treat the cause of the respiratory failure as aggressively as possible and to wean the child from mechanical ventilation as quickly as possible. If a child’s condition necessitates long-term mechanical ventilation, if an artificial airway is needed to bypass an upper airway obstruction, or if there is a risk for aspiration, a tracheostomy is usually performed.126
Infants and Children with Cardiovascular and Pulmonary Dysfunction
Cardiovascular and Pulmonary Development
Cardiac Development
Fetal Circulation
Neonatal Circulation
Closure of Foramen Ovale
Closure of Ductus Arteriosus
Pulmonary Development
Newborn Respiration
Respiration in the Child
Cardiovascular and Pulmonary Considerations in the Preterm Infant
Anatomical
Physiological
Capillary beds not well developed before 26 weeks of gestation
Increased pulmonary vascular resistance leading to right-to-left shunting
Type II alveolar cells and surfactant production not mature until 35 weeks of gestation; elastic properties of lung not well developed; lung space decreased by relative size of the heart and abdominal distention
Decreased lung compliance
Decreased responsiveness of ductus arteriosus to oxygen tensions; delayed ductal closure
Left-to-right shunting
Type I, high-oxidative fibers compose only 10% to 20% of diaphragm muscle
Diaphragmatic fatigue: respiratory failure
Highly vascular subependymal germinal matrix not resorbed until 35 weeks of gestation, increasing the vulnerability of the infant to hemorrhage
Decreased or absent cough and gag reflexes; apnea
Lack of fatty insulation and high surface area to body/weight ratio
Hypothermia and increased oxygen consumption
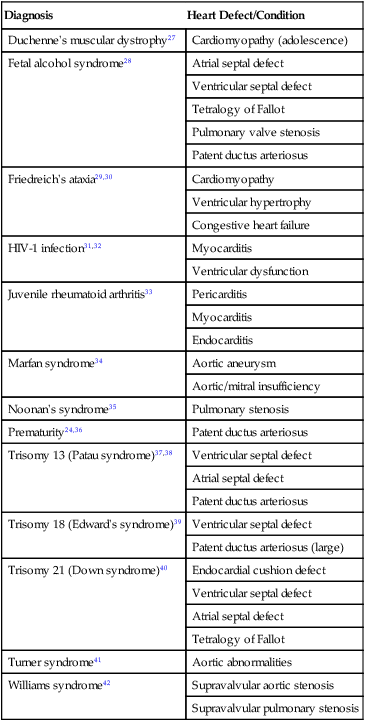
Common Pediatric Diagnoses
Cardiac Diagnoses
Diagnosis
Heart Defect/Condition
Duchenne’s muscular dystrophy27
Cardiomyopathy (adolescence)
Fetal alcohol syndrome28
Atrial septal defect
Ventricular septal defect
Tetralogy of Fallot
Pulmonary valve stenosis
Patent ductus arteriosus
Friedreich’s ataxia29,30
Cardiomyopathy
Ventricular hypertrophy
Congestive heart failure
HIV-1 infection31,32
Myocarditis
Ventricular dysfunction
Juvenile rheumatoid arthritis33
Pericarditis
Myocarditis
Endocarditis
Marfan syndrome34
Aortic aneurysm
Aortic/mitral insufficiency
Noonan’s syndrome35
Pulmonary stenosis
Prematurity24,36
Patent ductus arteriosus
Trisomy 13 (Patau syndrome)37,38
Ventricular septal defect
Atrial septal defect
Patent ductus arteriosus
Trisomy 18 (Edward’s syndrome)39
Ventricular septal defect
Patent ductus arteriosus (large)
Trisomy 21 (Down syndrome)40
Endocardial cushion defect
Ventricular septal defect
Atrial septal defect
Tetralogy of Fallot
Turner syndrome41
Aortic abnormalities
Williams syndrome42
Supravalvular aortic stenosis
Supravalvular pulmonary stenosis
Patent Ductus Arteriosus
Endocardial Cushion Defects and Atrioventricular Septal Defects
Tetralogy of Fallot
Pulmonary Diagnoses
Respiratory Distress Syndrome
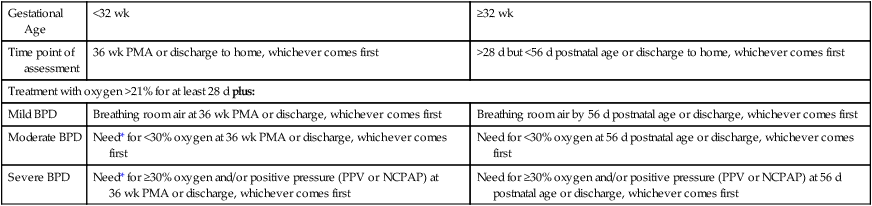
Bronchopulmonary Dysplasia
Gestational Age
<32 wk
≥32 wk
Time point of assessment
36 wk PMA or discharge to home, whichever comes first
>28 d but <56 d postnatal age or discharge to home, whichever comes first
Treatment with oxygen >21% for at least 28 d plus:
Mild BPD
Breathing room air at 36 wk PMA or discharge, whichever comes first
Breathing room air by 56 d postnatal age or discharge, whichever comes first
Moderate BPD
Need* for <30% oxygen at 36 wk PMA or discharge, whichever comes first
Need for <30% oxygen at 56 d postnatal age or discharge, whichever comes first
Severe BPD
Need* for ≥30% oxygen and/or positive pressure (PPV or NCPAP) at 36 wk PMA or discharge, whichever comes first
Need for ≥30% oxygen and/or positive pressure (PPV or NCPAP) at 56 d postnatal age or discharge, whichever comes first
Transient Tachypnea of the Newborn
Meconium Aspiration Syndrome
Pneumonia
Pneumocystis Jiroveci Pneumonia in Children Infected with HIV
Asthma
Cystic Fibrosis
Special Respiratory Problems Associated with Intubation and Tracheostomy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Infants and Children with Cardiovascular and Pulmonary Dysfunction
