Anemia and major bleeding are independent predictors of outcomes after acute coronary syndromes and percutaneous coronary intervention (PCI). Although the transradial approach reduces the incidence of bleeding, the hemoglobin changes after transradial PCI have not been defined. We serially assessed the hemoglobin values before and after transradial PCI and evaluated the effect of hemoglobin changes on outcomes. In the EArly Discharge After Transradial Stenting of CoronarY Arteries (EASY) trial, 1,348 patients underwent transradial PCI. All patients received aspirin, clopidogrel, and a bolus of abciximab before PCI. The hemoglobin values were assessed immediately before and 4 to 6 hours and 12 to 24 hours after PCI. The major adverse cardiac events (death, myocardial infarction, and target vessel revascularization) were assessed ≤3 years after PCI. According to the World Health Organization classification, 206 patients (15%) had anemia before PCI and 410 (30%) developed anemia within 24 hours after PCI. A mean hemoglobin decrease of 0.6 ± 1.0 g/dl occurred within 24 hours after PCI. At 30 days, the major adverse cardiac events were significantly increased when the hemoglobin decrease within 24 hours after PCI was >3 g/dl (p = 0.0002). Patients with anemia within 24 hours after PCI had significantly more major adverse cardiac events at 30 days, 6 months, 1 year, and 3 years than patients without anemia (log-rank p = 0.0044). After adjustment for differences in the baseline characteristics, anemia within 24 hours after PCI remained an independent predictor of major averse cardiac events at 3 years (hazard ratio 1.30, 95% confidence interval 1.01 to 1.67, p = 0.045). In conclusion, within 24 hours after transradial PCI with maximal antiplatelet therapy, only a mild hemoglobin decrease was observed. The choice of a hemoglobin decrease >3 g/dl after PCI as a cutoff value for current definitions of major bleeding in modern PCI trials appears reasonable. Measures to prevent anemia and blood loss during PCI remain to be further studied.
Transradial percutaneous coronary intervention (PCI) is associated with a lower risk of access site complications and bleeding than with the femoral approach. The clinical effect of bleeding after PCI has been increasingly recognized. Major bleeding after PCI is a strong independent predictor of mortality after femoral and transradial PCI. However, over the years, the definitions of major and minor bleeding have evolved with different cutoffs for the hemoglobin decrease. Recent efforts have led to the development of effective and new antithrombotic strategies such as fondaparinux before PCI and bivalirudin during PCI to maintain ischemic protection and reduce the incidence of access site and nonaccess site bleeding complications, at least with the femoral approach. Anemia in acute coronary syndromes and after coronary artery bypass grafting (CABG) confers a worse prognosis. However, few data have been reported on the hemoglobin or hematocrit changes after PCI using the femoral approach, and no data have been published for the transradial approach. We assessed the incidence, range, and clinical effects of hemoglobin changes after transradial coronary stenting and maximal antiplatelet therapy in the EArly Discharge After Transradial Stenting of CoronarY Arteries (EASY) trial ( Clinicaltrial.gov identifier no. NCT00169819 ).
Methods
The details of the EASY trial have been previously described. Owing to the study design, a contraindication for same-day discharge such as ST-segment elevation myocardial infarction (MI) within 72 hours and history of left ventricular ejection fraction of ≤30% or a contraindication for abciximab administration were the exclusion criteria. The Health Canada and Laval Hospital ethics review board approved the protocol. All patients provided written informed consent for participation in the trial.
The study was a randomized, controlled, open-label study comparing same-day home discharge and bolus-only of abciximab (n = 504) with overnight hospitalization and a bolus dose followed by a 12-hour infusion of abciximab (n = 501) after uncomplicated transradial coronary stenting. In the case of angiographic or clinical complications, the patients were excluded from same-day discharge after PCI and received the abciximab bolus and infusion (n = 343). Abciximab was administered as a 0.25 mg/kg bolus before the first balloon angioplasty and infusion was given for a total of 12 hours at 0.125 μg/kg/min to a maximum of 10 μg/min. All patients were pretreated with aspirin and clopidogrel before diagnostic angiography. After radial or ulnar sheath insertion, a bolus of 70 U/kg heparin was given intravenously with a target final activated clotting time of 300 seconds. The vascular sheaths were removed at the end of the procedure, and a bracelet (Hemostop, Zoom, Piedmont, Quebec, Canada) remained in place until hemostasis was completed, usually within 2 hours. Cardiac biomarkers and complete blood counts were evaluated on blood samples collected immediately before the procedure, shortly after PCI (4 to 6 hours), and the next day (12 to 24 hours). Study personnel interviewed all patients the day after PCI and at 30 days, 180 days, and 1 and 3 years after PCI.
The major cardiac adverse event (MACE) rate, including death, MI, and target vessel revascularization was calculated at 30 days and 6, 12, and 36 months after the index procedure. The end point adjudications and classifications at all points were made by a clinical events committee who was unaware of the study groups. Revascularization, including repeat PCI or CABG, was also assessed. For non–Q-wave MIs, periprocedural MIs were classified when any post-PCI creatine kinase MB value was ≥3 times the upper limit of normal (i.e., 30 μg/ml at our laboratory). After hospital discharge, non–Q-wave MIs were classified using the American College of Cardiology/European Society of Cardiology nomenclature (i.e., using any troponin-I or troponin-T-value or creatine kinase/creatine kinase-MB values greater than the upper limits of normal. Major bleeding episodes were categorized using the Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical events-2 (REPLACE-2) trial classification. Anemia was categorized using the World Health Organization criteria (i.e., hemoglobin <13 g/dl for men and hemoglobin <12 g/dl for women). All blood samples were analyzed in the same core laboratory. At 3 years of follow-up, vital status data were complete for 100%, and the MACE rate for 99.2% of the study population.
Categorical variables are presented as numbers and percentages and continuous variables as the mean ± SD. The baseline and procedural characteristics were compared using Fischer’s exact test or chi-square test for categorical variables and Student’s t test for continuous variables. Potential predictors of anemia at 24 hours after PCI were selected with stepwise, backward, and forward procedures with logistic regression analyses. Survival curves were constructed using Kaplan-Meier techniques, and comparisons between anemic and nonanemic patients after PCI were done using the log-rank test. The Cox proportional hazard model was used to assess the relative risks of anemia 24 hours after PCI on clinical outcomes for ≤3 years. Stepwise selection was used to identify potential predictors, which were entered into the model at p <0.20 and retained at p <0.10. p Values <0.05 were considered significant. Statistical tests were performed using JMP, version 7.0 (SAS Institute, Cary, North Carolina).
Results
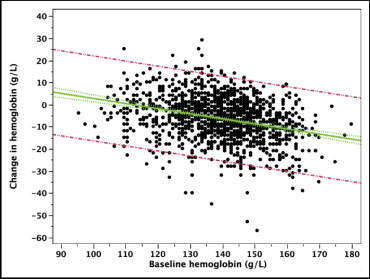
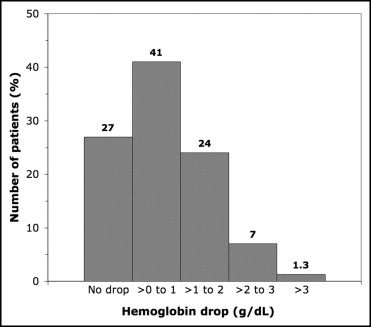
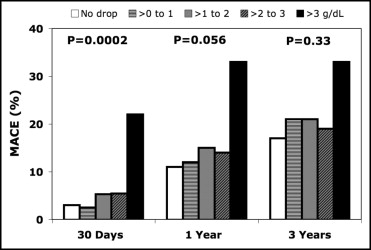
Of the 1,348 patients enrolled in the trial, 206 (15%) presented with anemia before PCI and 410 (30%) met the World Health Organization criteria for anemia within 24 hours after PCI ( Tables 1 and 2 ). Significantly more women had anemia than men both before (23% vs 13%, p <0.0001) and after PCI (44% vs 26%, p <0.0001). Overall, a mean hemoglobin decrease of 0.6 ± 1.0 g/dl occurred within 24 hours after PCI ( Figure 1 ). The hematocrit decreased from 41.1% to 38.1% at 4 to 6 hours after PCI and to 39.0% the next day. As shown in Figure 2 , 27% had no hemoglobin decrease and 41% had ≤1 g/dl, 24% a decrease of 1 to 2 g/dl, 7% a decrease of 2 to 3 g/dl, and 1.3% a decrease of >3 g/dl. Considering the incidence of MACE according to the hemoglobin decrease, a clear cutoff was seen with a significant increase in MACE at 30 days when the hemoglobin decrease was >3 g/dl ( Figure 3 ). This effect progressively vanished during follow-up.
| Characteristic | Anemia 24 Hours After PCI | p Value | |
|---|---|---|---|
| Yes (n = 410; 30%) | No (n = 938; 70%) | ||
| Age (years) | 64 ± 11 | 59 ± 10 | <0.0001 |
| Men | 278 (68%) | 772 (82%) | <0.0001 |
| Diabetes mellitus | 96 (23%) | 137 (15%) | <0.0001 |
| Previous myocardial infarction | 193 (47%) | 406 (43%) | 0.21 |
| Previous percutaneous coronary intervention | 76 (19%) | 185 (20%) | 0.65 |
| Acute coronary syndrome | 293 (71%) | 610 (65%) | 0.023 |
| Previous coronary artery bypass surgery | 35 (8.5%) | 50 (5.3%) | 0.029 |
| Glycoprotein IIb/IIIa inhibitors before percutaneous coronary intervention | 31 (7.6%) | 36 (3.8%) | 0.0060 |
| Anemia before percutaneous coronary intervention | 167 (41%) | 39 (4.2%) | <0.0001 |
| Platelet count (10 9 /L) | 245 ± 64 | 236 ± 57 | 0.018 |
| Hematocrit (%) | 38.0 ± 3.5 | 42.5 ± 3.3 | <0.0001 |
| Creatinine clearance (ml/min) | 81 ± 29 | 96 ± 41 | <0.0001 |
| Weight (kg) | 77 ± 17 | 83 ± 16 | <0.0001 |
| Characteristic | Anemia 24 Hours After PCI | p Value | |
|---|---|---|---|
| Yes (n = 410; 30%) | No (n = 938; 70%) | ||
| Number of vessels | |||
| 1 | 227 (55%) | 573 (61%) | 0.073 |
| 2 | 136 (33%) | 287 (31%) | |
| 3 | 47 (11%) | 78 (8.3%) | |
| Number of sites dilated | |||
| 1 | 245 (60%) | 598 (64%) | 0.17 |
| 2 | 115 (28%) | 255 (27%) | |
| ≥3 | 50 (12%) | 85 (9.0%) | |
| ≥1 B2/C lesion | 277 (68%) | 517 (55%) | <0.0001 |
| Largest catheter sheath used | |||
| 5Fr | 160 (39%) | 468 (50%) | 0.0002 |
| 6Fr | 244 (60%) | 466 (50%) | |
| 7Fr | 6 (1.5%) | 4 (0.4%) | |
| Final activated clotting time (s) | 310 ± 65 | 311 ± 66 | 0.83 |
| Compromised/suboccluded branch | 20 (4.9%) | 36 (3.8%) | 0.38 |
| Thrombolysis In Myocardial Infarction flow <3 after stenting | 9 (2.2%) | 18 (1.9%) | 0.83 |
| Procedure duration (min) | 55 ± 30 | 46 ± 23 | <0.0001 |
Patients with anemia at 24 hours after PCI had a gradual hemoglobin decrease at 4 to 6 hours after PCI and 24 hours after PCI. In contrast, nonanemic patients had only a transient hemoglobin decrease at 4 to 6 hours after PCI, which had almost completely recovered at 24 hours ( Figure 4 ). Of the independent predictors of anemia at 24 hours, anemia before PCI was the strongest independent factor (odds ratio 13.5, 95% confidence interval 9.17 to 20.41, p <0.0001; Figure 5 ). Patients with anemia within 24 hours after PCI had significantly more MACE at 30 days, 6 months, 1 year, and 3 years than did patients without ( Figures 6 and 7 ). Although mortality did not significantly differ between the patients with and without anemia within 24 hours after PCI, patients with anemia had significantly more MI and target vessel revascularization than did patients without anemia at all intervals ( Figure 6 ). Furthermore, although no difference was found in the rates of repeat PCI at 1 year (9% vs 7%, p = 0.28) or at 3 years (14% vs 13%, p = 0.67) in patients with and without anemia within 24 hours after PCI, significantly more CABG had occurred at 1 year (5% vs 2%, p = 0.0086) and 3 years (6% vs 3%, p = 0.0023) in the group of patients with anemia after PCI. On multivariate analysis, anemia within 24 hours after PCI remained an independent predictor of MACE ≤3 years after PCI ( Table 3 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


