The mechanisms responsible for late and very late stent thrombosis remain incompletely understood. This study aimed to evaluate the incidence and morphologic predictors of intrastent thrombus in patients after drug-eluting stent (DES) implantation using optical coherence tomography (OCT). A total of 208 patients with 262 DES who underwent follow-up OCT examination >6 months after DES implantation were included. The detailed vascular morphology including characteristics of neointima was analyzed. Thrombus was identified in 24 patients (11.5%) 11 months after DES implantation. Minimal lumen cross-sectional area was significantly smaller in the thrombus group than in the nonthrombus group (2.9 ± 1.7 vs 4.6 ± 2.0 mm 2 ; p <0.001). No difference was found in the frequency of uncovered or malapposed struts between the 2 groups. Thin-cap fibroatheroma (20.6% vs 0.1%; p <0.001) and heterogeneous neointima (22.2% vs 9.0%; p = 0.001) were more frequently detected in the thrombus group compared to the nonthrombus group. Second-generation DES showed lower incidence of thrombus, uncovered struts, and extrastent lumen compared with first-generation DES. In conclusion, the present OCT study revealed that smaller lumen cross-sectional area and neointimal morphology are important factors associated with intrastent thrombus. Second-generation DES demonstrated improved arterial healing and a lower incidence of intrastent thrombus compared with first-generation DES.
Recent angioscopy and optical coherence tomography (OCT) studies revealed that intracoronary thrombus is commonly detected in lesions treated with drug-eluting stents (DES). Although the clinical impact of such thrombus remains unclear, there is concern regarding a possible link between the presence of subclinical intrastent thrombus and late stent thrombosis (ST) risk. However, the incidence, determinants, and clinical impacts of intracoronary thrombus after DES implantation, especially in the era of new-generation DES, have not been systematically evaluated in real-world clinical practice. Therefore, the present study sought to evaluate the incidence and morphologic predictors of intrastent thrombus after DES implantation.
Methods
The Massachusetts General Hospital (MGH) OCT Registry is a multicenter registry of patients from 20 sites across 6 countries undergoing OCT imaging of the coronary arteries. Any patient who underwent an OCT procedure at a participating site was eligible for inclusion in the registry. For the present study, we identified 320 patients from the MGH OCT Registry with previously implanted DESs at the time of OCT imaging performed from August 2010 to January 2014. Of these patients, 112 patients were excluded for the following reasons: (1) 17 patients with poor OCT image quality; (2) 18 patients with incomplete demographic data; and (3) 77 patients in whom follow-up OCT imaging was performed <6 months after initial stent implantation. A total of 208 patients with 262 DES were therefore included in the final analysis.
At the time of OCT follow-up, 26 patients presented with acute coronary syndrome, 6 patients presented with stable angina, and 176 patients were asymptomatic. The selection of DES at the time of coronary intervention was at the discretion of the physician. The 262 DES examined in this study comprised 135 sirolimus-eluting stents (SES), 14 paclitaxel-eluting stents (PES), 22 zotarolimus-eluting stents (ZES), 70 everolimus-eluting stents (EES), and 21 biolimus-eluting stents (BES). First-generation DES included SES and PES; second-generation DES included ZES, EES, and BES. DES implantation was performed using conventional techniques. Dual-antiplatelet therapy (aspirin and clopidogrel) was recommended to all patients for ≥12 months after DES implantation. The study protocol was approved by the institutional review board at each site, and written informed consent was obtained from all patients. The MGH OCT Registry is registered on ClinicalTrials.gov ( NCT01110538 ).
Coronary angiograms were analyzed using offline software (CAAS 5.10.1; Pie Medical Imaging BV, Maastricht, Netherlands). Diameter stenosis, reference diameter, and minimum lumen diameter were measured.
OCT imaging in the present study was performed using either a time-domain OCT (TD-OCT) system (M2/M3 Cardiology Imaging System; LightLab Imaging, Inc, Westford, Massachusetts) or a frequency-domain OCT (FD-OCT) system (C7-XR OCT Intravascular Imaging System; St Jude Medical, St. Paul, Minnesota). The technique of intracoronary OCT imaging has been previously described. All OCT images were stored digitally, deidentified, and submitted to the MGH laboratory for offline analysis.
Cross-sectional OCT images were analyzed at 1-mm intervals. All cross-sectional images were initially screened for image quality and excluded from analysis if a side branch occupied >45° of the cross section or if there was excessive artifact related to residual blood or reverberation. For quantitative analysis, stent and lumen cross-sectional areas (CSAs) were measured, and neointimal hyperplasia (NIH) CSA was calculated as the stent CSA minus the lumen CSA. Percent NIH CSA was calculated as NIH CSA × 100/stent CSA. The thickness of NIH was measured as the distance between the endoluminal surface of the neointima and the strut. An uncovered strut was defined when no material covering a strut was found. A malapposed strut was defined as a strut that was detached from the vessel wall (SES ≥160 μm, PES ≥130 μm, ZES ≥110 μm, EES ≥100 μm, and BES ≥130 μm). Malapposition distance was measured from the center of the strut blooming to the adjacent lumen border. The maximal malapposition distance was recorded for each cross section. The percentages of uncovered or malapposed struts in each stent was calculated as the (number of uncovered or malapposed struts/total number of struts in each stent) × 100, respectively.
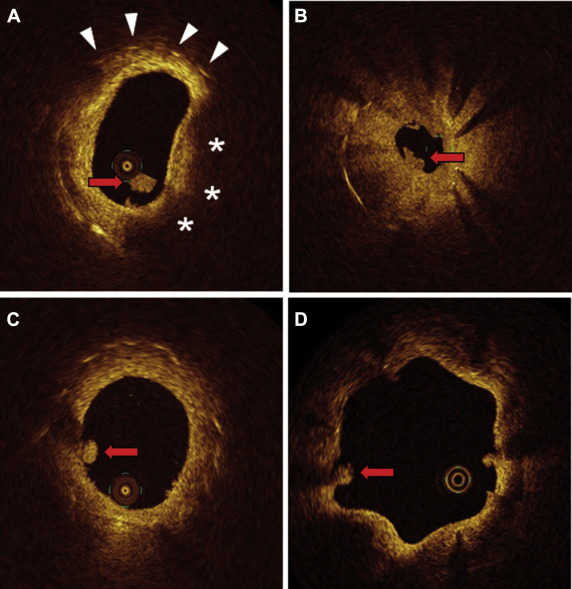
For qualitative analysis, thrombus was identified as any abnormal mass protruding into the vessel lumen, discontinuous from the surface of the vessel wall and with a dimension ≥250 μm. To differentiate thrombus from neointimal dissection or plaque protrusion, we excluded protruding masses <250 μm in maximal thickness and those without surface irregularity. Neointimal tissue was classified as follows: (1) homogeneous neointima, a uniform signal rich band without focal variation or attenuation; (2) heterogeneous neointima, variable optical properties and backscattering patterns; and (3) lipid-laden neointima, a diffusely bordered, signal-poor region with marked attenuation. Thin-cap fibroatheroma (TCFA) was defined by lipid-rich neointima with cap thickness ≤65 μm and an angle of lipidic tissue >90°. Neovascularization was defined as a signal-poor hole or tubular structure with a diameter ≥50 μm and ≤300 μm that was present on at least 3 consecutive frames. Extrastent lumen (ESL) was defined as outward bulges created by the luminal contour in the vessel wall between struts. Representative images are shown in Figure 1 .
OCT images were analyzed at the MGH OCT core laboratory by 2 independent investigators blinded to patient information. When there was discordance between the readers, a consensus reading was obtained from a third independent investigator.
Categorical data were expressed as counts and percentages and compared using a chi-square test or Fisher’s exact test, depending on the distribution of the data. Continuous measurements were expressed as mean ± standard deviation and analyzed with the Student t test. Comparison between groups and determination of the independent predictors for the presence of intrastent thrombus were performed by multivariate logistic modeling for which the inference was carried out using generalized estimating equations in consideration of the within-subject correlation because of multiple stents analyzed within a single patient. Multivariate regression models included the parameters that showed statistical significance with p <0.05 in the univariate analysis. Factors entered into the multivariate model included stent generation, stent diameter, minimum luminal CSA, frequency of uncovered struts, maximal malapposition distance, neointimal morphology, and ESL. Interobserver and intraobserver reliabilities were estimated by means of the κ coefficient for binary outcomes. All statistical analyses were performed with SPSS 17.0 (SPSS Inc, Chicago, Illinois). A 2-tailed p value of <0.05 was considered statistically significant.
Results
Intrastent thrombus was detected in 24 (11%) of 208 patients. The mean follow-up duration between DES implantation and follow-up OCT study was 11.5 ± 5.2 months in the thrombus group and 10.0 ± 4.4 months in the nonthrombus group (p = 0.160). A comparison of patient characteristics between the thrombus group and nonthrombus group is summarized in Table 1 . Stent diameter, reference vessel size, and minimal luminal diameter at follow-up were significantly smaller in the thrombus group compared with the nonthrombus group ( Table 2 ).
| Characteristic | Coronary Thrombus | p value | |
|---|---|---|---|
| Yes (n = 24) | No (n = 184) | ||
| Age (years) | 57.0 ± 11.8 | 60.6 ± 10.1 | 0.105 |
| Men | 19 (79%) | 129 (70%) | 0.475 |
| Hypertension | 13 (54%) | 118 (64%) | 0.373 |
| Hyperlipidemia | 19 (79%) | 134 (73%) | 0.738 |
| Smoker | 13 (54%) | 100 (54%) | 1.000 |
| Chronic kidney disease | 1 (4%) | 6 (3%) | 0.582 |
| Diabetes mellitus | 8 (33%) | 69 (37%) | 0.823 |
| Left ventricular ejection fraction | 63.6 ± 7.0 | 62.4 ± 8.2 | 0.471 |
| Prior myocardial infarction | 6 (25%) | 49 (27%) | 1.000 |
| Prior coronary bypass | 0 (0) | 1 (0.5%) | 1.000 |
| Clinical presentation at stenting | 0.393 | ||
| Acute coronary syndrome | 9 (37%) | 87 (47%) | |
| Stable angina pectoris | 15 (63%) | 97 (53%) | |
| Clinical presentation at follow-up | |||
| Acute coronary syndrome | 4 (17%) | 22 (12%) | 0.513 |
| Stable angina pectoris | 2 (8%) | 4 (2%) | 0.144 |
| Medications at follow-up | |||
| Aspirin | 23 (96%) | 179 (97%) | 0.525 |
| Clopidogrel | 18 (75%) | 139 (75%) | 1.000 |
| Dual antiplatelet therapy | 17 (71%) | 134 (73%) | 0.837 |
| Statin | 22 (92%) | 171 (92%) | 0.686 |
| ACEI / ARB | 6 (25%) | 57 (31%) | 0.642 |
| β-Blockers | 10 (42%) | 58 (31%) | 0.358 |
| Characteristic | Coronary Thrombus | p value | |
|---|---|---|---|
| Yes (n = 26 DESs) | No (n =236 DESs) | ||
| Location of coronary stent | 0.901 | ||
| Left anterior descending | 14 (54%) | 117 (50%) | |
| Left circumflex | 5 (19%) | 46 (19%) | |
| Right | 7 (27%) | 73 (31%) | |
| Stent diameter (mm) | 2.7 ± 0.3 | 2.9 ± 0.4 | 0.002 |
| Stent length (mm) | 23.1 ± 7.7 | 22.1 ± 7.2 | 0.483 |
| Stent type | 0.081 | ||
| Sirolimus-eluting stent | 17 (65%) | 118 (50%) | |
| Paclitaxel-eluting stent | 3 (11%) | 11 (5%) | |
| Zotarolimus-eluting stent | 3 (11%) | 19 (8%) | |
| Everolimus-eluting stent | 3 (11%) | 67 (28%) | |
| Biolimus-eluting stent | 0 (0) | 21 (9%) | |
| QCA at Follow-up | |||
| Diameter stenosis (%) | 29.3 ± 15.9 | 17.0 ± 10.7 | < 0.001 |
| Minimum lumen diameter (mm) | 1.8 ± 0.6 | 2.4 ± 0.5 | < 0.001 |
| Reference diameter (mm) | 2.5 ± 0.4 | 2.8 ± 0.4 | < 0.001 |
A total of 4,285 struts in 26 DESs with intrastent thrombus and 32,167 struts in 236 DESs without thrombus were analyzed. Quantitative OCT analyses are provided in Table 3 . Minimal lumen CSA and minimal stent CSA were significantly smaller, and percent NIH CSA was significantly greater in the thrombus group compared with the nonthrombus group. The frequencies of malapposed strut per stent, uncovered struts per stent, and cross sections with uncovered strut ratios >0.3 were similar between the 2 groups. On qualitative evaluation of neointimal morphology, lipid-laden neointima (42.3% vs 16.5%; p = 0.004), TCFA (20.6% vs 0.1%; p <0.001), and heterogeneous neointima (22.2% vs 9.0%; p = 0.001) were more frequently detected in the thrombus group compared to the nonthrombus group. The incidence of ESL and neovascularization was also significantly higher in the thrombus group ( Figure 2 ).
| Variable | Coronary Thrombus | p Value | |
|---|---|---|---|
| Yes (n = 26 DESs) | No (n = 236 DESs) | ||
| Stent level analysis | |||
| Minimum lumen CSA (mm 2 ) | 2.9 ± 1.7 | 4.6 ± 2.0 | < 0.001 |
| Minimum stent CSA (mm 2 ) | 4.8 ± 2.0 | 5.8 ± 2.0 | 0.010 |
| Mean neointima area (mm 2 ) | 1.4 ± 0.7 | 1.0 ± 0.7 | 0.008 |
| Percent NIH CSA (%) | 24.7 ± 12.9 | 15.4 ± 10.7 | < 0.001 |
| Strut level analysis | |||
| Number of struts analyzed/stent | 164.8 ± 66.7 | 136.3 ± 52.7 | 0.036 |
| Mean Neointimal thickness (mm) | 0.2 ± 0.1 | 0.1 ± 0.1 | < 0.001 |
| Uncovered struts (%) | 4.9 ± 6.7 | 4.2 ± 5.4 | 0.575 |
| Cross sections with uncovered struts > 0.3 (%) | 4.7 ± 9.5 | 3.5 ± 8.0 | 0.507 |
| Maximum length of uncovered segment (mm) | 1.7 ± 2.1 | 1.7 ± 1.8 | 0.956 |
| Malapposed strut (%) | 1.5 ± 2.5 | 0.8 ± 2.1 | 0.116 |
| Maximum malapposition distance (mm) | 0.2 ± 0.2 | 0.1 ± 0.2 | 0.072 |
| Maximum length of malapposed segment (mm) | 0.9 ± 1.5 | 0.4 ± 0.7 | 0.063 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


