Drug
Nutritional effect
Anticonvulsants:
Folate, Ca, Vit B12, B6, D, C. Dyslipidemias
Phenytoin
Folate, Ca, Vit B12, B6, K, C. Dyslipidemias
Phenobarbital
Anti-inflammatories:
Vit C, Fe
Aspirin
Proteins, fats, glucose, Zn, K, Na, Ca
Corticosteroids
Antimicrobials:
↓Endogenous Vit K in the bowels
Isoniazid
Vit B6, niacin
Trimethoprim
Folate
Penicillin
K
Cathartics:
Vit A, D, E, K, carotene, Ca
Mineral oil
Others:
K, Mg, Ca
Digitalis
Zn, Ca, Na, K
Furosemide
Ca, Vit A, Fe, phosphate
Antiacids
B12, Fe
H2 antagonists
Fe, Ca
Inhibitor b. Protons
Vit A, D, E, K, Ca, fats
Cholestyramine
Beta-agonists
↑GERD in infants, K
Mechanisms Involved in Malnutrition
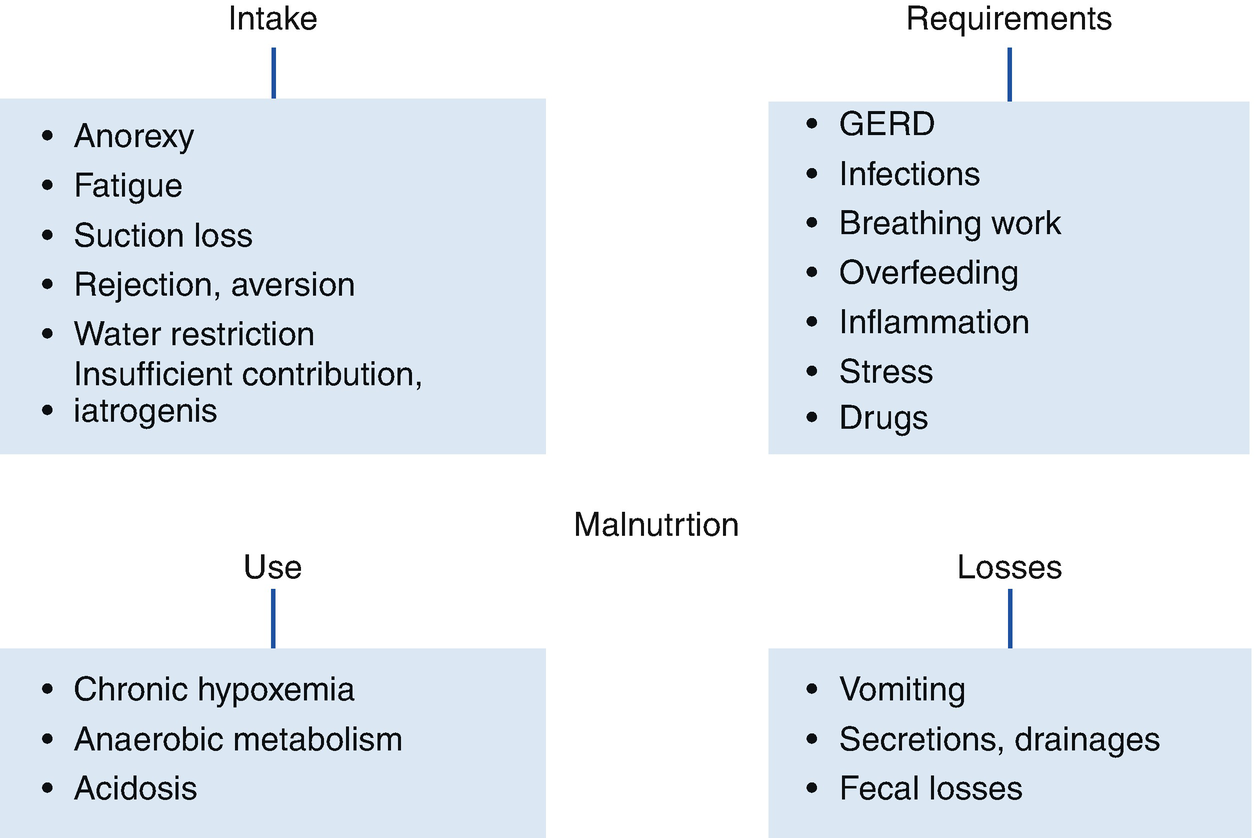
Mechanisms of malnutrition in children with chronic respiratory diseases
Anorexia caused by chronic diseases can increase with acidosis, drugs, infections, and specific deficits (iron or zinc, the latter producing dysgeusia). Food intake is also affected by infant’s fatigue, water restriction in those with bronchopulmonary dysplasia, alteration of swallowing maturation processes due to immaturity and prolonged use of probes, and the development of behavioral disorders. Moreover, frequent hospitalizations are associated with lower intake and even setbacks concerning achieved milestones.
Resting metabolic rate (RMR) increase: It has been demonstrated in children with bronchopulmonary dysplasia, cystic fibrosis, and in those with steroid treatment. Infection, inflammation or stress increase energy requirements, anorexia, and low weight. Although the work of breathing constitutes only 2–3% of the BMR, in preterm infants its impact is greater. Excessive inputs can increase TDEE, although normally only 10% corresponds to the DIT. This occurs especially if the excess is protein, whose storage expense is greater than that of carbohydrates (and greater than that of fat). In children with bronchopulmonary dysplasia, a high load of glucose via parenteral administration increases breathing work, with less effect if enterally administered.
Anaerobic metabolism: It has lower energy efficiency; chronic hypoxemia, even mild (during sleep or feeding), affects growth. Moreover, acidosis decreases protein accretion and exacerbates anorexia.
Digestive losses: These increase with vomiting associated with cough, especially in infections or worsening of the disease. Gastroesophageal reflux is common in children with bronchopulmonary dysplasia, neurological diseases, or who are tube-fed. In addition, dysphagia and esophagitis cause a decrease in intake due to pain. In cystic fibrosis there are intestinal losses due to steatorrhea and, also, there may be protein loss through secretions or drainages (bronchiectasis, cystic fibrosis).
Interaction Between Nutrition and Lung Function
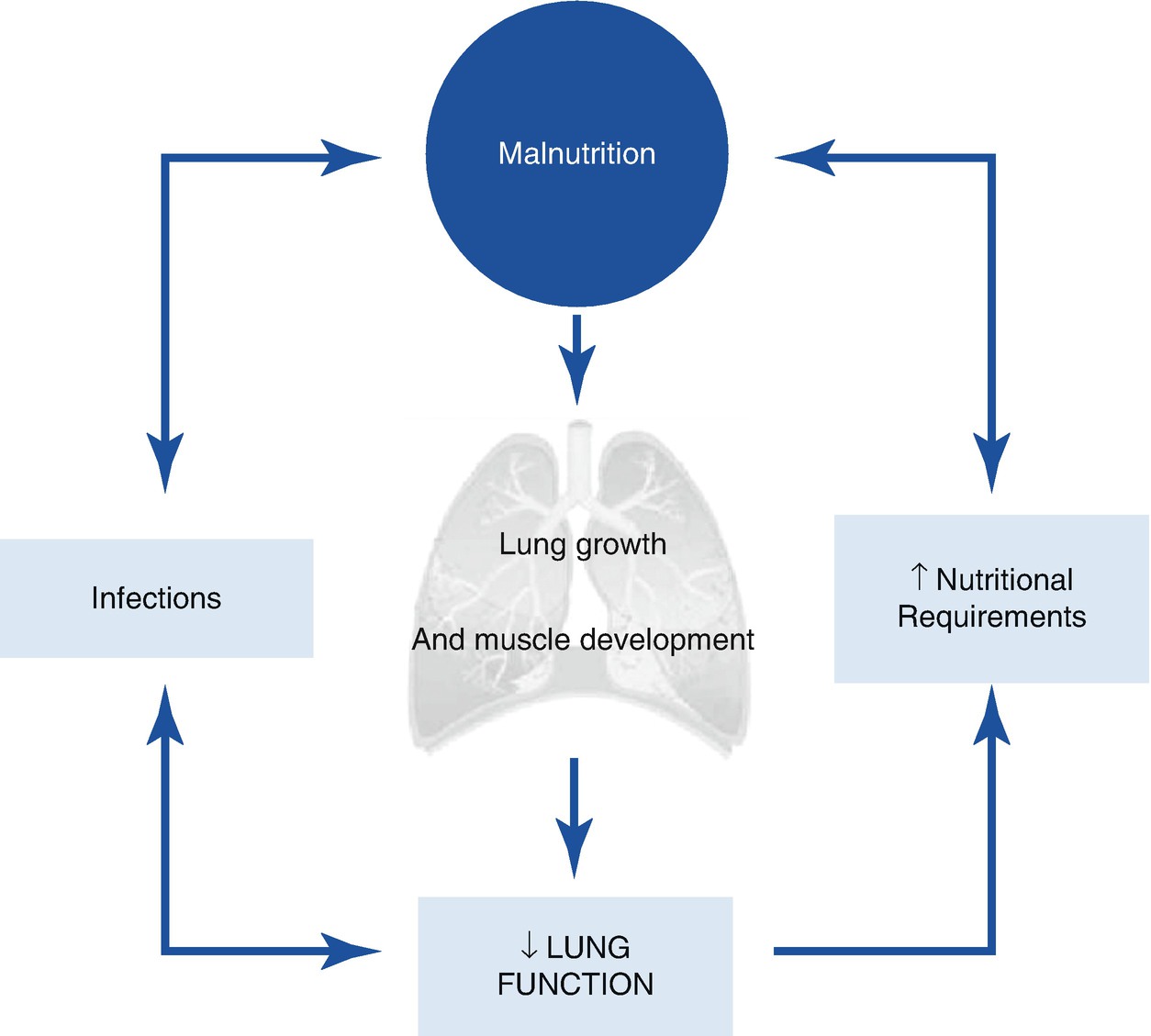
Interaction between nutrition and lung function
It has been suggested that in preterm infants malnutrition begins in prenatal life, so it precedes the development of bronchopulmonary dysplasia and interacts later with other factors, decreasing the lung regeneration mechanism, the defense against hyperoxic damage, the response to infections, and lung growth. Nutritional improvement promotes a better evolution of the underlying disease and improvement in lung damage contributes to optimize the nutritional status.
Malnutrition by excess can also affect lung function and defense mechanisms. Although in children nutritional deficit continues to determine the prognosis, this would be worse in adults with severe obesity who suffer from acute pulmonary infections, as it was demonstrated in the epidemic of influenza A (H1N1).
Specific Diseases
Bronchopulmonary Dysplasia
Preterm patients who develop bronchopulmonary dysplasia are a group at high risk of malnutrition. The factors of prematurity or intrauterine growth restriction are added to those of the disease during a period of high demand and accelerated growth. In the period immediately following neonatal discharge, malnutrition ranges between 30% and 67%. Subsequently, infants have accelerated weight gain until the sixth month, followed by deceleration. At 1 year corrected age, they have a weight-for-age Z-score (ZW/A) of −1.5 and −2.7 (male and female). Regarding height, they progress at a normal rate, but in smaller numbers and reach height-for-age (ZH/A) of −0.8 and −1.5 a year. However, they have lower lean mass and lower total body fat after the sixth month than term infants. The impact of pre and postnatal corticosteroids on height and bone mineral accretion has been recognized, although its use is justified considering the benefit–cost ratio.
The greatest nutritional deficiency in children with bronchopulmonary dysplasia occurs within their first three years of life; in the neonatal period, low nutritional intake is caused predominantly by delay in initiating and achieving enteral supply, high requirements, volume restriction, and comorbidity. These patients usually develop suction–swallowing disorders, gastroesophageal reflux, and subclinical hypoxemia, recurrent infections or obstructive episodes, with repeated hospitalizations. However, long-term growth would be affected to a greater extent by factors derived from prematurity, in comparison to factors related to bronchopulmonary dysplasia.
The prevention of bronchopulmonary dysplasia by nutritional modification or supplementation is controversial and is related to protection against oxidative damage. There is greater evidence of the preventive role of vitamin A, which promotes re-epithelialization and tissue repair. Its use in preterm infants <1000g was linked with a lower occurrence of death or O2 requirement at 1 month of age (RR 0.75–0.93), a trend similar to 36 weeks of gestational age, with decreased retinopathy. This was not linked with a better long-term prognosis and although other deleterious effects have not been demonstrated, the most effective route is painful and the dose is high (intramuscular, 5000 IU, 3 times per week for 4 weeks). More studies are required, especially if it coexists with corticosteroid therapy that increases plasma levels of retinol. The evidence is scarce for the protective role of vitamin E and polyunsaturated fatty acids, and insufficient for inositol, selenium, and magnesium.
Preterm infants, with or without bronchopulmonary dysplasia, have a higher risk of developing recurrent wheezing. The influence of pre or postnatal factors, within which the development of obesity is found, has been studied. Also, preterm infants have a higher risk of chronic diseases and higher cardiovascular risk in adulthood. It has been suggested that the intrauterine programming of those with low birth weight (in particular, small for gestational age), accentuated by the accelerated gain of postnatal weight, are significant factors that would explain it. For both reasons, it is advisable that once the initial period of nutritional deficit is over, excess contributions are avoided and a healthy diet and an active lifestyle are stimulated, preventing a sedentary lifestyle that is frequent in these patients.
Post-infectious Chronic Lung Damage
Nutritional compromise usually develops later after a variable period of normal growth. However, growth can be significantly affected if malnutrition is premature, leading to a delayed growth in height. Thus, in a group of 18 Brazilian children with post-viral chronic lung damage, oxygen-dependent, 4.5 ± 2.7 years, 47% had short stature (Z-score for H/A ≤2). This is not necessarily irreversible: in a group of 21 Chilean children with severe bronchiolitis obliterans and similar height deficits, it was possible to achieve growth and height recovery with adequate management. However, in those patients in which there is no such recovery and have less physical activity, the development of obesity is also promoted, so that they require adequate follow-up and nutritional advice.
Cystic Fibrosis
Cystic fibrosis constitutes a model of interaction between chronic lung disease and nutritional status. Along with improvement of survival, it has been possible to observe the impact of the nutritional status optimization on the prognosis, without considering malnutrition as an unavoidable condition. The corresponding chapter discusses the multidisciplinary approach that several consensuses have delineated to anticipate and modulate the different nutritional variables that intervene in its treatment.
Asthma
There is controversy about the relationship between obesity and asthma. As both are multifactorial in nature, it is difficult to demonstrate common causality, which is discussed in the corresponding chapter. In clinical and epidemiological studies there is a higher prevalence of obesity in asthma. A balanced diet, decreased sedentary lifestyle, and increased physical activity are the most valuable tools to prevent and treat obesity in these patients.
Patients with Prolonged Ventilatory Support
Children who require chronic ventilatory support have different nutritional problems according to their underlying disease; those with severe chronic respiratory diseases tend to develop protein–caloric malnutrition, and patients with neuromuscular diseases may present malnutrition due to deficit or excess. Malnutrition affects respiratory function, since it reduces muscle mass and contractile force, which results in decreased respiratory effort, endurance, and vital capacity. These effects are, in general, reversible with nutritional improvement. Chronic ventilatory support in infants promotes nutritional progression and, conversely, it is possible to confirm in clinical practice that the withdrawal of ventilatory support may be associated with lower weight development and should be done gradually, monitoring oxygenation, and in parallel to a greater nutritional contribution if required.
Some children with neurological diseases and chronic ventilatory support may have higher caloric expenditure, such as those with hypertonia, severe seizure syndromes or increased movements, but most of them have decreased muscle mass (more active metabolic tissue) combined with less mobility and growth. This determines a lower energy requirement, so that proper contributions for their sex, age, and weight can lead to a greater fat mass deposition in most of them, although not necessarily reflected in the rates of overweight or obesity. This increases immobility, breathing work, loss of bone mass, and impairs motor and respiratory rehabilitation. Thus, energy requirement must be corrected by the muscle tone and degree of physical activity. However, the excessive limitation of the contribution in order to prevent obesity can lead to protein, mineral, and trace element depletion, so there must be monitoring and follow-up by an multi-disciplinary team that including a nutrition specialist.
Nutritional Support In Chronic Respiratory Diseases
The main objective of the nutritional support in children with chronic respiratory diseases is to optimize their growth and development, to promote a better evolution of their disease and quality of life, as well as to prevent the chronic disease of adulthood.
The following describes the nutritional management of children with chronic lung damage in the period after discharge from the neonatal unit, in the case of preterm infants with bronchopulmonary dysplasia. The nutritional treatment during the hospital stay can be reviewed in the references, and that of children with cystic fibrosis or asthma, in the corresponding chapter. Some general principles of nutritional support can be considered first, and specific management measures can be considered later.
Regular Monitoring of Food Intake
Because one of the main causes of poor weight gain in patients with chronic respiratory diseases is low intake, it is important to make a detailed nutritional history, considering the previous and present dietary history: feeding schedules, composition of milk formula and meals, method of preparation, use of supplements, volume offered and received, feeding time, and respiratory and gastrointestinal tolerance. In older children, one should ask for food between meals and general habits. Food records of 2 or 3 days a week provide very useful objective information.
Regular Monitoring of Nutritional Status
It is necessary to monitor growth, since the sequential measurement of the improvement in weight, height, and cranial circumference allows early identification of the deceleration or lack of improvement. It is essential to graph the evolution of the particular patient, which allows clear visualization of the tendency in its growth. In preterm infants it is recommended to adjust the chronological age up to 2 years and in cases of children of less than 28 weeks, up to 3 years.
Weight/age (W/A) is the most sensitive index in detecting malnutrition, being able to overestimate it in those with low genetic or family size.
Height/age (H/A) is less sensitive than W/A, and it is more strongly affected in periods of rapid growth; indicates long-term nutritional status.
Weight/height (W/H) is sensitive, although it underestimates the deficit in children with short stature. It indicates an adequacy of the weight for the height, a relation that can also be expressed as Waterlow index (%) = (current weight/ideal weight) × 100.
Body mass index (BMI) also expresses the harmony between weight and height and it is usually considered after 2 years of life. In cystic fibrosis, a close correlation between BMI and pulmonary function has been demonstrated, which guides the nutritional management.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


