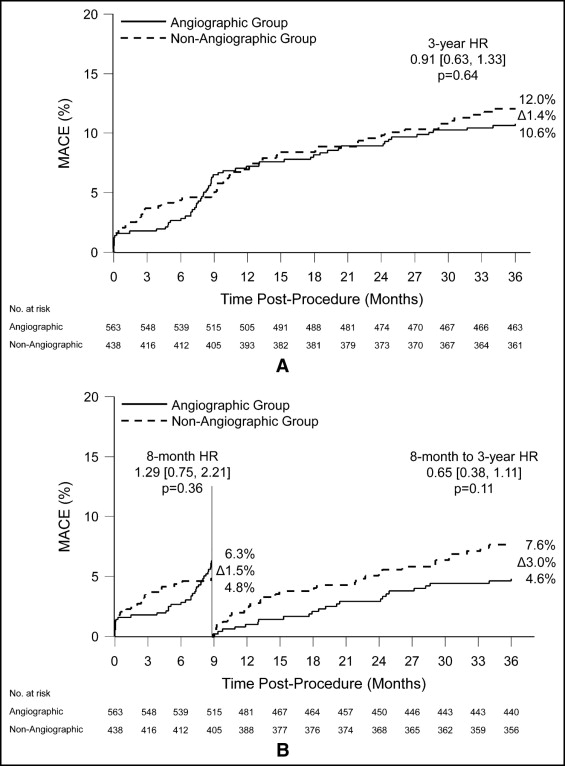
Routine angiographic follow-up after bare-metal stent implantation has been associated with an increase in coronary revascularization. The impact of angiographic follow-up after drug-eluting stent placement remains poorly characterized. The prospective, randomized, single-blinded SPIRIT III trial assigned patients to the everolimus-eluting stent or the paclitaxel-eluting stent (PES). Major adverse cardiovascular events (cardiac death, myocardial infarction, and ischemia-driven target lesion revascularization [ID-TLR]) at 3 years were assessed by angiographic versus clinical-only follow-up at 8 months ± 28 days and a landmark survival analysis from 9 months to 3 years. Of 1,002 patients, 564 patients were assigned to angiographic follow-up at 8 months ± 28 days and 438 patients underwent clinical follow-up alone. Three-year major adverse cardiovascular event rates were 10.6% in the angiographic group and 12.0% in the clinical follow-up group (p = 0.64). Ischemia-driven revascularization increased twofold at 9 months, but no difference was noted in ID-TLR for either device. Non–ID-TLR was significantly higher in patients in the angiographic group (4.5% vs 1.0%, p = 0.002), a difference resulting from PES (9.1% vs 0.7%, p = 0.0007) rather than everolimus-eluting stent (2.2% vs 1.1%, p = 0.36) treatment. The landmark analysis showed no significant differences between the angiographic and clinical follow-up groups from 9 months to 3 years of major clinical outcomes. In conclusion, routine angiographic follow-up in SPIRIT III did not increase rates of ID-TLR compared to clinical follow-up alone. Despite higher nonischemia-driven revascularization rates with angiographic follow-up of patients with PESs, none of the safety end points were adversely affected.
The benefits of percutaneous coronary intervention compared to balloon angioplasty have been tempered by restenosis. Although bare-metal stents (BMSs) decreased the risk of restenosis, the introduction of drug-eluting stents (DESs) resulted in a marked 60% to 80% decrease in restenosis compared to balloon angioplasty and BMSs. Despite the widespread adoption of DESs, controversy remains on the clinical impact of protocol-mandated angiographic follow-up in DES clinical trials, which may preferentially accentuate rates of repeat revascularization and negatively affect safety outcomes. Follow-up angiography has been associated with exaggerated rates of revascularization, ostensibly resulting from the “oculostenotic reflex,” a term describing revascularization using percutaneous coronary intervention owing to anatomic lesion severity independent of clinical or physiologic evidence of ischemia. Although this phenomenon is well recognized using balloon angioplasty and BMSs, few investigations have studied the impact of routine angiographic follow-up with DESs. This study aimed to assess the impact of protocol-mandated angiographic follow-up in patients enrolled in A clinical Evaluation of the Investigational Device XIENCE V Everolimus Eluting Coronary Stent System (EECSS) in the Treatment of Subjects With de Novo Native Coronary Artery Lesions (SPIRIT III) trial.
Methods
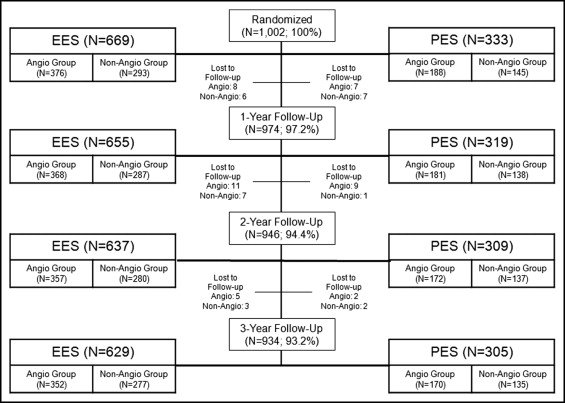
The SPIRIT III trial was a prospective, multicenter, randomized, controlled, single-blinded trial in which 1,002 patients were randomized in a 2:1 ratio to the everolimus-eluting stent (EES; XIENCE V, Abbott Vascular, Santa Clara, California) or the paclitaxel-eluting stent (PES; TAXUS Express , Boston Scientific, Natick, Massachusetts). In brief, patients with 1 de novo lesion or 2 de novo coronary lesions with reference vessel diameters of 2.5 to 3.75 mm and lesions ≤28 mm in length were eligible for randomization. Telephone randomization was performed in blocks of 3 and 6 patients stratified by diabetes status, 2-vessel treatment, and study site. EESs were available in 2.5-, 3.0-, and 3.5-mm diameters with lengths of 8, 18, and 28 mm. PESs were available in 2.5- to 3.5-mm diameters and lengths ranging from 8 to 32 mm. Operators were instructed to choose a stent length to cover approximately 3 mm of nondiseased tissue at the proximal and distal stent edges. When multiple stents were necessary, 1 to 4 mm of stent overlap was recommended. Operators were not blinded to randomization; however, patients and staff involved in follow-up assessments remained blinded throughout follow-up. The protocol-mandated angiographic follow-up was specified at 8 months for the first 564 randomized patients in the study. A landmark analysis was performed from the last day of the 8th-month angiographic follow-up window (268 days) or from 9 months to 3 years.
Clinical end points used in the SPIRIT III trial have been previously defined. In brief, major adverse cardiovascular events were defined as the composite of cardiac death, myocardial infarction, and ischemia-driven target lesion revascularization (ID-TLR). Myocardial infarction was defined as the development of new pathologic Q waves ≥0.4 second in duration in ≥2 contiguous leads or as an increase in creatinine phosphokinase-MB. Revascularization was categorized as ID or non- ID. ID revascularization required (1) a positive functional study, or (2) the presence of symptoms and a >50% diameter stenosis by independent core laboratory, or (3) in the absence of symptoms or a positive functional study required >70% diameter stenosis by an independent core laboratory. ID revascularization was further adjudicated by the angiographic core laboratory based on the location of recurrence as ID-TLR and ID target vessel revascularization (non-TLR or revascularization remote from the original target site). Non-ID-TLR was defined as revascularization in the absence of any of the previously described criteria.
Protocol-defined stent thrombosis was (1) an acute coronary syndrome with angiographic evidence of thrombus within or adjacent to a previously treated lesion, or (2), in the absence of angiographic evidence, any unexplained death or ST-segment elevation myocardial infarction, or (3) new Q waves in the distribution of the target lesion within 30 days of the index percutaneous coronary intervention. Stent thrombosis was also independently adjudicated according to the Academic Research Consortium definition. All clinical events were adjudicated by an independent clinical events committee (Harvard Clinical Research Institute).
All baseline, follow-up, and event-related angiograms were analyzed by an independent angiographic core laboratory (Cardiovascular Research Foundation, New York, New York) by analysts blinded to treatment allocation using standard definitions and methods for all quantitative measurements using Medis software (Medis, Leiden, the Netherlands). All outcomes are reported at 3 years from the index percutaneous coronary intervention and the 9-month (268 days, upper window of the 8th month) landmark date and are based on the intent-to-treat principle.
The cohort was divided into patients undergoing protocol-mandated angiography at 8 months and those undergoing standard clinical follow-up. During the index percutaneous coronary intervention, each patient was treated with an EES or a PES. Landmark survival analysis was used to determine whether there were differences in the primary outcome between routine angiographic and standard clinical follow-up groups after 268 days (last day of 8th month angiographic follow-up window) or the 9-month landmark. Patients in the standard clinical follow-up group who died, developed myocardial infarction, or underwent repeat revascularization within 268 days of the index percutaneous coronary intervention were excluded from the landmark analysis. Similarly, patients in the routine angiographic group who died, developed myocardial infarction, or underwent repeat revascularization before the scheduled angiographic follow-up were excluded from the landmark analysis.
Continuous variables are reported as mean ± SD and were compared by t test. Categorical variables are reported as absolute values and percentages with comparisons made with the Fisher exact test. The Kaplan–Meier method was used to construct survival curves for the primary end point of a major adverse cardiovascular event (cardiac death, myocardial infarction, or ID-TLR) and its components. Comparisons between groups were made with log-rank test. Cox proportional hazards models were used to generate hazard ratios and 95% confidence intervals for the study outcomes. The proportionality assumption of the proportional hazards model was assessed and satisfied using log(−log) plots. All tests are 2-tailed, with differences reported as statistically significant if the p value was <0.05. All analyses were performed with SAS 9.1 (SAS Institute, Cary, North Carolina).
Results
The SPIRIT III trial enrolled 1,002 patients at 65 United States sites from June 2005 through March 2006. Patients were randomized to receive EESs (n = 669) or PESs (n = 333). In total 564 randomized patients were assigned to undergo coronary angiography at 8 months. Of these, 435 (77%) underwent angiography as planned at 240 ± 28 days or had a previous angiogram that qualified for analysis. Angiographic and clinical follow-up groups were well matched for cardiac risk factors, location, and severity of coronary artery disease ( Table 1 ) and for procedural and angiographic characteristics ( Table 1 ).
| Angiographic Group | Nonangiographic Group | p Value | |
|---|---|---|---|
| (n = 564) | (n = 438) | ||
| Baseline characteristics | |||
| Demographics | |||
| Age (years), mean ± SD | 63.3 ± 10.4 | 62.8 ± 10.4 | 0.41 |
| Men | 387/563 (68.7%) | 300/438 (68.5%) | 0.95 |
| Hypertension | 419/562 (74.6%) | 336/438 (76.7%) | 0.46 |
| Hypercholesterolemia | 400/554 (72.2%) | 323/431 (74.9%) | 0.35 |
| Diabetes mellitus | |||
| Any | 160/562 (28.5%) | 29.7% (130/437) | 0.67 |
| Requiring insulin | 38/56 (26.8%) | 7.3% (32/437) | 0.80 |
| Smoker, current | 121/551 (22.0%) | 106/433 (24.5%) | 0.36 |
| Target vessel | |||
| Left anterior descending coronary artery | 269/647 (41.6%) | 212/503 (42.1%) | 0.86 |
| Left circumflex coronary artery | 182/647 (28.1%) | 138/503 (27.4%) | 0.84 |
| Right coronary artery | 196/647 (30.3%) | 151/503 (30.0%) | 0.95 |
| Left main coronary artery (protected) | 0/647 (0.0%) | 2/503 (0.4%) | 0.19 |
| Number of narrowed coronary arteries | |||
| 1 | 378/563 (67.1%) | 276/438 (63.0%) | 0.18 |
| 2 | 136/563 (24.2%) | 117/438 (26.7%) | 0.38 |
| ≥3 | 49/563 (8.7%) | 44/438 (10.0%) | 0.51 |
| American College of Cardiology/America Heart Association lesion class | |||
| A | 44/640 (6.9%) | 35/50 (17.0%) | 1.00 |
| B1 | 223/640 (34.8%) | 188/501 (37.5%) | 0.35 |
| B2 | 243/640 (38.0%) | 199/501 (39.7%) | 0.58 |
| C | 130/640 (20.3%) | 79/501 (15.8%) | 0.05 |
| Thienopyridine use | |||
| At 180 days | 524/559 (93.7%) | 411/432 (95.1%) | 0.41 |
| At 270 days | 408/549 (74.3%) | 337/426 (79.1%) | 0.09 |
| At 365 days | 372/543 (68.5%) | 315/417 (75.5%) | 0.02 |
| At 730 days | 277/515 (53.8%) | 263/410 (64.1%) | 0.002 |
| At 1,095 days | 253/512 (49.4%) | 225/402 (56.0%) | 0.05 |
| Procedural and angiographic characteristics | |||
| Procedural variables, mean ± SD | |||
| Number of lesions | 647 | 508 | |
| Number of stents per patient | 1.3 ± 0.6 | 1.3 ± 0.6 | 0.38 |
| Number of stents per lesion | 1.2 ± 0.4 | 1.1 ± 0.4 | 0.09 |
| Maximum stent diameter per lesion (mm) | 3.01 ± 0.37 | 3.03 ± 0.39 | 0.31 |
| Total stent length per lesion (mm) | 22.4 ± 8.0 | 22.3 ± 8.6 | 0.89 |
| Maximum pressure (atm) | 14.8 ± 2.8 | 15.0 ± 2.8 | 0.28 |
| Glycoprotein IIb/IIIa inhibitors used | 150/563 (26.6%) | 116/438 (26.5%) | 1.00 |
All patients received a thienopyridine at discharge ( Table 1 ), with a shorter mean duration of therapy in the angiographic compared to the clinical follow-up group (682 ± 398 vs 750 ± 376 days, p = 0.006, respectively). Thienopyridine use was similar at 6 months (180 days) between groups ( Table 1 ). At 9 months, 1 year, 2 years, and 3 years, clopidogrel use was greater in the clinically followed group. In contrast, aspirin use was similar between the angiographic and clinical follow-up groups at all time points, with a mean duration of aspirin therapy of 999 ± 241 days in the angiographic group and 997 ± 253 days in the clinical follow-up group (p = 0.92).
Follow-up at 3 years was available in 934 patients (93.2% of study cohort), with similar follow-up rates between groups ( Table 2 , Figure 1 ) . There was no significant difference in 3-year major adverse cardiovascular events between the angiographic and clinical follow-up groups ( Figure 2 ) , and the composite of cardiac death and myocardial infarction was also similar ( Figure 3 ) . There were no differences between the 2 groups in myocardial infarction, Academic Research Consortium definite or probable stent thrombosis, or bleeding complications ( Table 2 ).
| Result/Outcome | Angiographic Group | Nonangiographic Group | p Value |
|---|---|---|---|
| (n = 564) | (n = 438) | ||
| Cardiac death | 9/522 (1.7%) | 7/412 (1.7%) | 1.00 |
| Myocardial infarction | 21/522 (4.0%) | 23/412 (5.6%) | 0.28 |
| Q wave | 2/522 (0.4%) | 3/412 (0.7%) | 0.66 |
| Non-Q wave | 19/522 (3.6%) | 20/412 (4.9%) | 0.41 |
| Stent thrombosis (Academic Research Consortium) | |||
| Definite | 4/523 (0.8%) | 4/408 (1.0%) | 0.74 |
| Probable | 2/523 (0.4%) | 3/408 (0.7%) | 0.66 |
| Definite or probable | 6/523 (1.1%) | 7/408 (1.7%) | 0.58 |
| Target lesion revascularization, all | 55/532 (10.3%) | 31/416 (7.5%) | 0.14 |
| Ischemia driven | 37/532 (7.0%) | 27/416 (6.5%) | 0.80 |
| Nonischemia driven | 24/532 (4.5%) | 4/416 (1.0%) | 0.002 |
| Target vessel revascularization (nontarget lesion revascularization), all | 49/532 (9.2%) | 27/416 (6.5%) | 0.15 |
| Ischemia driven | 43/532 (8.1%) | 26/416 (6.3%) | 0.31 |
| Nonischemia driven | 7/532 (1.3%) | 3/416 (0.7%) | 0.53 |
| Bleeding complications | 23/515 (4.5%) | 25/405 (6.2%) | 0.30 |