The left main coronary artery (LMCA) is a particularly important target of atherosclerotic plaque accumulation. The aim of this study was to investigate the connection between subclinical plaque burden in the LMCA measured by intravascular ultrasound and future cardiovascular events. Two hundred eighteen consecutive patients underwent percutaneous coronary intervention for the left anterior descending coronary artery or the left circumflex coronary artery under intravascular ultrasound guidance. Plaque burden in the LMCA was analyzed for these patients, and major adverse cardiac events were also evaluated. Data were analyzed by grouping the patients into tertiles according to plaque burden values; tertile 1, <32% area stenosis; tertile 2, 32% to 45% area stenosis; and tertile 3, >45% area stenosis. During a 3-year follow-up period (average 16.1 months), 12% of tertile 1, 18% of tertile 2, and 40% of tertile 3 experienced major adverse cardiac events, mostly due to repeat revascularization (p <0.001). On Cox multivariate analysis, plaque burden in the LMCA (per percentage) detected by intravascular ultrasound remained an independent significant predictor of major adverse cardiac events (hazard ratio 1.04, 95% confidence interval 1.02 to 1.07) and future revascularization (hazard ratio 1.05, 95% confidence interval 1.02 to 1.07) (p <0.001). In conclusion, plaque burden in the LMCA is useful as an indicator of coronary atherosclerosis and may be a significant predictor of cardiovascular events, especially revascularization.
Although the left main coronary artery (LMCA) is a particularly important target of atherosclerotic plaque accumulation, the relation between percentage area stenosis of the LMCA measured by intravascular ultrasound (IVUS) and cardiovascular events has hitherto not been evaluated in detail. IVUS allows cross-sectional imaging of coronary arteries and thus can provide more comprehensive assessment of atherosclerotic plaque information in vivo. As a consequence, IVUS is a useful tool of the assessment of coronary atherosclerosis. In early coronary disease, angiographic results often remain normal or show trivial irregularities, and IVUS provides a better indication of atheroma accumulation. Therefore, in the present study, we assessed the impact of coronary plaque with angiographically ambiguous LMCA lesions clarified using IVUS on adverse cardiac events in patients with cardiovascular disease, taking into account possible confounding factors.
Methods
From April 2003 to December 2005, we prospectively recruited patients who underwent percutaneous coronary intervention (PCI) for the de novo left anterior descending coronary artery or left circumflex coronary artery lesions. Exclusion criteria were defined as follows: (1) calcifications limiting quantitative assessment of vessel cross-sectional area (CSA); (2) LMCA lesions with severe stenosis, defined as >50% diameter stenosis assessed by quantitative coronary angiography; and (3) having undergone PCI for LMCA. In total, 232 LMCA lesions in 232 consecutive patients were enrolled in this study. Of these patients, 14 were excluded because of an inability to perform IVUS (2 patients) and to evaluate IVUS images (12 patients). Ultimately, 218 LMCA lesions in 218 consecutive patients were evaluated. We analyzed the smallest luminal area within their LMCA lesions using IVUS. All patients provided written informed consent, and the study was approved by the hospital ethics committee.
At baseline, all patients underwent an interview by trained interviewers and laboratory tests. Standardized questionnaires assessed coronary risk factors, symptoms, history, and medications. Self-reports of myocardial infarction, angina pectoris, and congestive heart failure were confirmed and validated by review of medical records and relevant examinations. We defined major adverse cardiac events (MACEs) as all deaths including cardiac death, nonfatal myocardial infarction, any revascularization including PCI and coronary artery bypass grafting for new and/or restenosis lesions, and unplanned hospital admission for congestive heart failure. Hospitalization for congestive heart failure was defined as admission to the hospital or attendance at an acute health care facility for the administration of intravenous diuretic treatment, escalation of diuretic doses, and/or inotropes with or without confirmation of the diagnosis on chest x-ray. Revascularizations for the significant stenosis lesions at baseline were excluded from MACEs. The narrowed coronary artery was defined as the one with ≥50% stenosis in a vessel ≥1.5 mm angiographically. Follow-up data were obtained from hospital charts and telephone interviews with patients.
Coronary angiograms were obtained in multiple views after intracoronary injections of isosorbide dinitrate 2.5 mg. Quantitative coronary angiography analysis was performed with a validated edge detection system (MEDIS Medical Imaging Systems, Leiden, The Netherlands) by an experienced observer who was blinded to the clinical course. The reference vessel diameter, minimal luminal diameter, and percentage diameter stenosis were measured from the single worst view.
Global left ventricular function was determined from end-diastolic and end-systolic left ventricular endocardial contours using the centerline method and the global ejection fraction was determined as (end-diastolic volume − end-systolic volume)/end-diastolic volume × 100. The left ventricular ejection fraction was measured before PCI. The measurements were made by an experienced observer who was not aware of the clinical course.
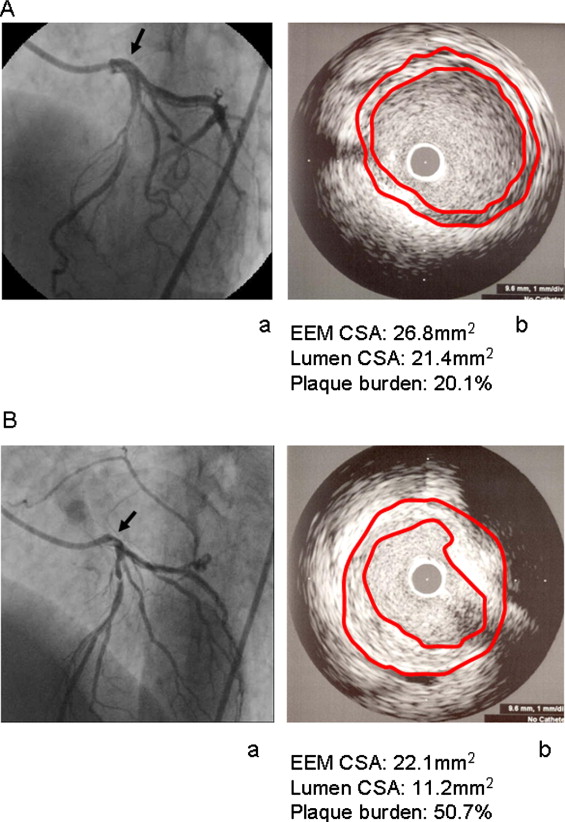
IVUS studies were performed with commercially available systems: a mechanical sector scanner and a 40-MHz transducer (Boston Scientific Corporation, Natick, Massachusetts). Slow, continuous pullback of the IVUS transducer was started in 1 of the left coronary arteries and was generally achieved using a motorized pullback device (at 0.5 mm/s). IVUS images during the entire pullback were recorded on 0.5-inch high-resolution S-VHS tapes or DVDs. The LMCA target site was the smallest luminal CSA within the LMCA plaque. If there were several slices with equal luminal areas, the slice with the largest external elastic membrane and plaque-plus-media (external elastic membrane minus lumen) CSA was analyzed. Quantitative analysis was performed according to criteria of the American College of Cardiology clinical expert consensus document on IVUS as follows. The luminal CSA was measured by tracing the leading edge of the intima and the external elastic membrane CSA with reference to the leading edge of the adventitia. Plaque burden (as a percentage) was calculated as (plaque-plus-media CSA)/(external elastic membrane CSA) × 100%. LMCA lesions whose sizes were larger than the IVUS region of interest or whose lengths were too short to measure were excluded. Representative cases are illustrated in Figure 1 . All measurements were performed by 2 independent experienced observers who were blinded to the clinical course. The correlation of plaque burden measured by 2 physicians who conducted IVUS was r 2 = 0.85 (p <0.001). There were no complications related to IVUS studies.
We analyzed data by grouping into tertiles according to plaque burden values. Baseline characteristics and IVUS findings are reported as mean ± SD and as percentages, with p values calculated using 1-way analysis of variance or the chi-square test. Kaplan-Meier analysis, the log-rank test, and the Cox proportional-hazards model were used for assessment of the tertiles and plaque burden in the LMCA (per percentage). Concerning multivariate analysis, the following variables were included: those with p values <0.20 on univariate analysis, age, gender, and clinical syndrome of acute myocardial infarction or unstable angina pectoris. Analyses were performed using StatMate3 for Windows (ATMS Corporation, Tokyo, Japan). Statistical significance was set a priori at p <0.05.
Results
The average duration of follow-up was 16.1 ± 10.5 months. In this study population (n = 218), the average age was 67.1 ± 9.7 years. The population comprised 77% men. Rates for histories of hypertension, hyperlipidemia, diabetes mellitus, current smoking, previous myocardial infarction, and previous PCI or coronary artery bypass grafting were 67%, 64%, 31%, 37%, 25%, and 34% respectively. The clinical presentation was stable angina pectoris in 73%, unstable angina pectoris in 16%, and acute myocardial infarction in 11%. In IVUS findings of all patients, the average values for external elastic membrane CSA, luminal CSA, plaque-plus-media CSA, and plaque burden were 25 ± 6 mm 2 , 15 ± 5 mm 2 , 10 ± 4 mm 2 , and 39 ± 13%, respectively. The ranges of plaque burden for each tertile were as follows: tertile 1, <32% area stenosis; tertile 2, 32% to 45% area stenosis; and tertile 3, >45% area stenosis. The baseline characteristics, angiographic findings, and IVUS findings of each tertile are listed in Tables 1 to 3 . There were no differences in age, history of hypertension, hyperlipidemia, current smoking, myocardial infarction, PCI or coronary artery bypass grafting, number of narrowed coronary arteries, clinical syndrome, the left ventricular ejection fraction, and medication use among the tertiles. However, according to plaque burden in the LMCA, there was a significant difference in gender. Moreover, the proportion of patients with diabetes mellitus and histories of smoking tended to increase across the tertiles. With regard to the number of narrowed coronary arteries, there were no differences among the tertiles, but there tended to be more 2-vessel disease patients in tertile 3 than in those in tertile 1 (p = 0.063). Concerning reference vessel diameter, minimal luminal diameter, and percentage diameter stenosis, there were significant differences among the tertiles statistically.
| Variable | Tertile 1 | Tertile 2 | Tertile 3 | p Value |
|---|---|---|---|---|
| (n = 73) | (n = 73) | (n = 72) | ||
| Age (years) | 66.1 ± 10.6 | 68.5 ± 9.3 | 66.8 ± 9.2 | 0.31 |
| Men | 45 (62%) | 60 (82%) | 62 (86%) | <0.001 |
| Hypertension ⁎ | 46 (63%) | 50 (69%) | 49 (68%) | 0.74 |
| Hyperlipidemia † | 43 (59%) | 49 (67%) | 48 (67%) | 0.51 |
| Diabetes mellitus | 15 (21%) | 24 (33%) | 28 (39%) | 0.05 |
| Current smoking | 21 (29%) | 26 (36%) | 33 (46%) | 0.10 |
| Previous myocardial infarction | 18 (25%) | 22 (30%) | 14 (19%) | 0.33 |
| Previous PCI/coronary artery bypass grafting | 19 (26%) | 29 (40%) | 25 (35%) | 0.21 |
| Clinical syndrome | 0.63 | |||
| Stable angina pectoris | 58 (79%) | 52 (71%) | 50 (69%) | |
| Unstable angina pectoris | 8 (11%) | 12 (17%) | 14 (20%) | |
| Acute myocardial infarction | 7 (10%) | 9 (12%) | 8 (11%) | |
| Left ventricular ejection fraction (%) | 63.4 ± 14.0 | 64.2 ± 11.1 | 62.4 ± 11.1 | 0.82 |
| Medications | ||||
| Aspirin | 73 (100%) | 73 (100%) | 72 (100%) | 1 |
| Angiotensin-converting enzyme inhibitors | 7 (10%) | 10 (14%) | 14 (19%) | 0.23 |
| Angiotensin 1 receptor antagonists | 32 (44%) | 35 (48%) | 37 (51%) | 0.66 |
| β blockers | 12 (16%) | 9 (12%) | 10 (14%) | 0.77 |
| Calcium channel blockers | 36 (49%) | 47 (64%) | 42 (58%) | 0.18 |
| Diuretics | 7 (10%) | 6 (8%) | 9 (13%) | 0.68 |
| Nitrates | 26 (36%) | 20 (27%) | 19 (26%) | 0.41 |
| Statins | 41 (56%) | 47 (64%) | 43 (60%) | 0.60 |
⁎ Systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg.
† Low-density lipoprotein cholesterol level >140 mg/dl or need for medical treatment.
| Variable | Tertile 1 | Tertile 2 | Tertile 3 | p Value |
|---|---|---|---|---|
| (n = 73) | (n = 73) | (n = 72) | ||
| Number of narrowed coronary arteries | 0.20 | |||
| 1 | 45 (62%) | 36 (49%) | 30 (42%) | |
| 2 | 22 (30%) | 29 (40%) | 34 (47%) | |
| 3 | 6 (8%) | 8 (11%) | 8 (11%) | |
| Reference vessel diameter (mm) | 4.4 ± 0.6 | 3.9 ± 0.6 | 3.7 ± 0.7 | <0.001 |
| Minimal luminal diameter (mm) | 4.3 ± 0.6 | 3.7 ± 0.6 | 3.5 ± 0.7 | <0.001 |
| Diameter stenosis (%) | 4.1 ± 5.2 | 5.3 ± 5.2 | 7.7 ± 6.5 | 0.048 |
| Target lesion | ||||
| Left anterior descending coronary artery | 57 | 59 | 58 | 0.90 |
| Left circumflex coronary artery | 16 | 14 | 14 | |
| Use of drug-eluting stent | 38 (52%) | 43 (59%) | 34 (47%) | 0.37 |
| Variable | Tertile 1 | Tertile 2 | Tertile 3 | p Value |
|---|---|---|---|---|
| External elastic membrane CSA (mm 2 ) | 25 ± 6 | 25 ± 6 | 25 ± 6 | 0.90 |
| Luminal CSA (mm 2 ) | 20 ± 5 | 15 ± 4 | 12 ± 3 | <0.001 |
| Plaque-plus-media CSA (mm 2 ) | 6 ± 2 | 10 ± 2 | 14 ± 4 | <0.001 |
| Plaque burden (%) | 23 ± 5 | 38 ± 4 | 54 ± 7 | <0.001 |
Over the 3-year observation period, 12% of tertile 1, 18% of tertile 2, and 40% of tertile 3 experienced MACEs ( Table 4 ). No patients experienced MACEs because of atherosclerotic plaque progression to the LMCA. On Kaplan-Meier analysis, the proportion of MACE-free survivors was significantly lower in tertile 3 compared to tertiles 1 and 2 (log-rank p <0.001; Figure 2 ), because of revascularization (log-rank p <0.001; Figure 2 ). In particular, it was due to new lesions. However, the proportions of all death were similar across tertiles ( Figure 2 ). Results of univariate Cox hazard analysis for MACEs and revascularization are summarized in Tables 5 and 6 . The hazard ratios of plaque burden for MACE and revascularization were 1.04 (95% confidence interval [CI] 1.02 to 1.06, p <0.001) and 1.05 (95% CI 1.02 to 1.07, p <0.001), respectively. Reference vessel diameter, minimal luminal diameter, and percentage diameter stenosis by angiographic evaluation were not significantly associated with MACEs. After adjustment for gender, age, clinical syndrome of acute myocardial infarction or unstable angina pectoris, number of narrowed coronary arteries, and variables with p values <0.20 on univariate analysis, plaque burden in the LMCA remained an independent significant predictor of MACEs (hazard ratio 1.04, 95% CI 1.02 to 1.07, p <0.001) and future revascularization (hazard ratio 1.05, 95% CI 1.02 to 1.07, p <0.001) on multivariate Cox proportional hazard analysis ( Tables 5 and 6 ). The adjusted hazard ratios of other parameters (male gender, age, clinical syndrome of acute myocardial infarction or unstable angina pectoris, number of narrowed coronary arteries, and variables with p values <0.20 on univariate analysis) are listed in Tables 5 and 6 .
| Variable | Tertile 1 | Tertile 2 | Tertile 3 | p Value |
|---|---|---|---|---|
| (n = 73) | (n = 73) | (n = 72) | ||
| All MACEs | 9 (12%) | 13 (18%) | 29 (40%) | <0.001 |
| All deaths | 1 (1%) | 2 (3%) | 2 (3%) | 0.81 |
| Cardiovascular death | 1 (1%) | 1 (1%) | 1 (1%) | >0.99 |
| Nonfatal myocardial infarction | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Revascularization | 7 (10%) | 12 (14%) | 26 (36%) | <0.001 |
| New lesions | 3 (4%) | 6 (8%) | 19 (26%) | <0.001 |
| Restenosis lesions | 4 (6%) | 4 (6%) | 7 (10%) | 0.62 |
| Unplanned hospitalization for congestive heart failure | 1 (1%) | 1 (1%) | 1 (1%) | 0.60 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


