There are few data on the incidence and clinical outcomes of patients with atrial fibrillation (AF) treated in the era of percutaneous coronary intervention (PCI). We analyzed 30-day clinical outcomes in 3,307 consecutive patients with and without AF (sinus rhythm) undergoing PCI from January 2007 through December 2008 enrolled in a multicenter Australian registry. Periprocedural AF was present in 162 patients (4.9%). AF was associated with older age (74.1 ± 8.9 vs 63.9 ± 11.9 years, p <0.001), higher baseline serum creatinine (0.13 ± 0.14 vs 0.10 ± 0.13 mmol/L, p = 0.01), and lower left ventricular ejection fraction (49.5 ± 13.2% vs 53.4% ± 11.6%, p <0.001). Significantly more patients with AF had a history of heart failure and cerebrovascular and peripheral arterial diseases (p ≤0.01 for all comparisons). Periprocedural glycoprotein IIb/IIIa inhibitor (31.5% vs 31.4%, p = 0.98) and antithrombin use were not different between groups, but in-hospital bleeding complications were higher in patients with AF (5.0% vs 2.1%, p = 0.015). Fewer patients with AF received drug-eluting stents (p = 0.004). AF was associated with a greater than fourfold increase in 30-day mortality (9.9% vs 2.2%, p <0.0001) and readmission rates at 30 days (p = 0.01). Fewer patients with AF were on dual antiplatelet therapy at 30 days (86.3% vs 94.3%, p <0.0001), although 28.1% of patients with AF were on triple therapy (dual antiplatelet therapy plus oral anticoagulation). In conclusion, patients with periprocedural AF represent a very high-risk group. Excess 30-day morbidity and mortality after PCI may be due to the higher incidence of co-morbidities, bleeding complications, and suboptimal antiplatelet therapy.
The increased mortality risk attributable to atrial fibrillation (AF) in myocardial infarction (MI) treated with percutaneous coronary intervention (PCI) and AF in coronary artery disease is multifactorial. This increased hazard associated with AF may relate to differences in baseline patient characteristics, presence of left ventricular dysfunction, postprocedure antiplatelet and anticoagulant regimens, and risk of thromboembolism and bleeding. Our aim was to assess the impact of AF at time of PCI on in-hospital and short-term clinical outcomes in patients undergoing PCI for acute coronary syndromes (ACSs) and more stable clinical scenarios.
Methods
We analyzed inpatient and 30-day clinical outcomes in 3,307 consecutive patients with and without AF (sinus rhythm [SR]) treated with PCI from January 2007 through December 2008 and who were enrolled in a large multicenter Australian registry (Melbourne Interventional Group [MIG]). AF was defined as an irregular rhythm with rapid atrial fibrillatory waves that varied in shape, amplitude, and timing that was present at the commencement of the index PCI. Using this definition, periprocedural AF was documented in 162 patients (4.9%). These patients were compared to 3,145 patients who were not in AF (SR) at the commencement of PCI.
The MIG registry is a collaborative venture of interventional cardiologists practicing at 6 Australian tertiary referral hospitals that was designed to record data pertaining to all PCI procedures and to perform follow-up at 30 days and periodically thereafter. The MIG registry has been previously described in detail. Briefly, demographic, clinical, and procedural characteristics of consecutive patients undergoing PCI are prospectively recorded on case-report forms using standardized definitions for all fields. The registry is coordinated by the Centre of Cardiovascular Research and Education in Therapeutics, a independent research body within the Department of Epidemiology and Preventive Medicine, Monash University (Melbourne, Australia). An audit of 15 verifiable fields from 5% of randomly selected procedures at each institution is performed periodically by an investigator not affiliated with the institution; data accuracy was 97% on the most recent audit, which compares favorably to other large registries. The registry has been approved by the ethics committee in each participating hospital. “Opt-out” informed consent was obtained in all patients, as previously described.
The indication for the PCI was recorded and included stable angina, unstable angina (with no increase in blood biomarkers), non–ST-segment elevation MI (non-STEMI), and STEMI. The interventional strategy, stent selection, and antithrombotic therapy were left to the discretion of the operator in all procedures. Dual antiplatelet therapy was recommended in all cases where a stent was deployed.
In-hospital complications were recorded at the time of discharge or death. Thirty-day follow-up was conducted by telephone, and patient medical records were reviewed to verify events. All cardiac events were documented including death (all-cause mortality, cardiac mortality), MI, target lesion revascularization, target vessel revascularization, and the composite of major adverse cardiac events (death, MI, or target vessel revascularization). MI was defined as (1) an increase in creatine kinase or creatine kinase-MB ≥3 times the upper limit of normal and/or (2) significant ST-segment segment change, development of new Q waves in ≥2 contiguous electrocardiographic leads, or new left branch bundle block pattern.
Major bleeding was defined by a decrease in hemoglobin >3.0 g/dl and/or requiring transfusion. Causes of major bleeding were recorded as retroperitoneal, access site complications, gastrointestinal, and “others,” which included bleeding at other sites. Acute renal failure was defined by an increase of serum creatinine to >0.20 mmol/L (2.27 mg/dl, or 2 times the baseline creatinine level) or need for dialysis. Stroke was defined by the sudden onset of persistent loss of neurologic function caused by an ischemic or hemorrhagic event during or after PCI. Cardiogenic shock was defined by hypotension (systolic blood pressure <90 mm Hg for ≥30 minutes or needing supportive measures), evidence of end-organ hypoperfusion or a cardiac index <2.2 L/min/m 2 , and a pulmonary capillary wedge pressure ≥15 mm Hg.
Stent thrombosis was defined according to the Academic Research Consortium definitions of definite or probable. Only early (0 to 30 days) stent thrombosis was included in this analysis.
Continuous variables are expressed as mean ± SD, and categorical data are expressed as number (percentage). Continuous variables were compared using Student’s t test. Categorical variables were compared using Fisher’s exact or chi-square tests as appropriate. Independent predictors for 30-day clinical outcomes were determined using multiple logistic regression models for variables with a p value <0.10 in simple logistic regression models (25 clinical and procedural variables were analyzed). Event-free survival for the SR and AF groups was constructed using the Kaplan–Meier method and compared by log-rank test. All calculated p values were 2 sided, and a p value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS 12.0 (SPSS, Inc., Chicago, Illinois) for Windows (Microsoft, Redmond, Washington).
Results
Of the 3,307 consecutive patients/procedures enrolled from January 2007 through December 2008, 162 patients (4.9%) met the inclusion criterion of having AF at the time of the PCI procedure and were compared to 3,145 patients in SR.
The cohort with AF was significantly older and had more underlying co-morbidities including heart failure, renal failure, cerebrovascular disease, and peripheral arterial disease ( Table 1 ). Patients with AF had higher baseline serum creatinine and lower mean left ventricular ejection fraction than patients with SR ( Table 1 ). The distribution of traditional cardiovascular risk factors between the AF and SR groups was similar apart from fewer patients in the AF group being current smokers.
| Variable | SR | AF | p Value |
|---|---|---|---|
| (n = 3,145) | (n = 162) | ||
| Age (years) | 63.9 ± 11.9 | 74.1 ± 8.9 | <0.001 |
| Men | 2,396 (76.3%) | 114 (70.8%) | 0.11 |
| Women | 746 (23.7%) | 47 (29.2%) | 0.11 |
| Diabetes mellitus | 816 (26%) | 50 (30.9%) | 0.17 |
| Renal failure ⁎ | 161 (5.1%) | 19 (11.7%) | <0.001 |
| Serum creatinine (mmol/L) | 0.10 ± 0.13 | 0.13 ± 0.14 | 0.01 |
| Hypertension † | 2,093 (66.7%) | 119 (73.5%) | 0.07 |
| Dyslipidemia ‡ | 2,309 (73.8%) | 110 (68.3%) | 0.12 |
| Current smoker | 763 (24.4%) | 21 (13%) | 0.001 |
| Family history of coronary artery disease | 1,298 (41.7%) | 56 (35.2%) | 0.11 |
| Previous myocardial infarction | 979 (31.2%) | 59 (36.4%) | 0.16 |
| Previous coronary artery bypass graft surgery | 276 (8.8%) | 18 (11.2%) | 0.30 |
| Previous heart failure | 121 (3.9%) | 26 (16%) | <0.001 |
| Left ventricular ejection fraction (%) | 53.4 ± 11.6 | 49.5 ± 13.2 | <0.001 |
| Cerebrovascular disease | 200 (6.4%) | 29 (18%) | <0.001 |
| Peripheral arterial disease | 253 (8.1%) | 24 (14.8%) | 0.003 |
| Chronic obstructive pulmonary disease | 146 (4.6%) | 12 (7.4%) | 0.11 |
| Obstructive sleep apnea | 167 (5.3%) | 8 (5%) | 0.84 |
⁎ Serum creatinine level >0.20 mmol/L.
† History of hypertension diagnosed and treated with medication, systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg on ≥2 occasions, or current use of antihypertensive medication.
‡ History of dyslipidemia diagnosed and/or treated with medication or a fasting total cholesterol level >5.0 mmol/L, high-density lipoprotein level <1.0 mmol/L, or triglyceride level >2.0 mmol/L.
ACS as the indication for the index PCI was similar between the 2 groups, with approximately 30% of patients in the 2 groups undergoing PCI because of STEMI or non-STE-ACS ( Table 2 ). Also, similar numbers in the 2 groups had stable angina or had received thrombolytics. Periprocedural glycoprotein IIb/IIIa inhibitor use and antithrombin use were not different between groups. Fewer patients with AF compared to patients with SR received drug-eluting stents ( Table 2 ).
| Variable | SR | AF | p Value |
|---|---|---|---|
| (n = 3,145) | (n = 162) | ||
| ST-segment elevation myocardial infarction | 856 (27.2%) | 53 (32.7%) | 0.11 |
| Non–ST-segment elevation myocardial infarction | 893 (28.4%) | 42 (25.9%) | 0.11 |
| Unstable angina | 277 (8.8%) | 20 (12.3%) | 0.11 |
| Stable angina | 1,119 (35.6%) | 47 (29.0%) | 0.11 |
| Thrombolysis | 117 (13.7%) | 9 (17%) | 0.50 |
| Glycoprotein IIb/IIIa inhibitor | 987 (31.4%) | 51 (31.5%) | 0.98 |
| Unfractionated heparin or low-molecular-weight heparin | 3,083 (98%) | 161 (99.4%) | 0.22 |
| Percutaneous transluminal coronary angioplasty only | 131 (4.3%) | 6 (3.8%) | 0.76 |
| Stent | 2,940 (95.7%) | 153 (96.2%) | |
| Drug-eluting stent | 1,087 (34.6%) | 38 (23.5%) | 0.004 |
| Bare-metal stent | 2,058 (65.4%) | 124 (76.5%) | 0.004 |
In-hospital bleeding complications were higher in patients with AF (5.0% vs 2.1%, p = 0.015), but there was no difference reported across bleeding sites ( Table 3 ). Small numbers of patients in the 2 groups were given blood transfusions.
| SR | AF | p Value | |
|---|---|---|---|
| (n = 3,145) | (n = 162) | ||
| Bleeding complications | 65 (2.1%) | 8 (5%) | 0.015 |
| Bleeding site | |||
| Retroperitoneal | 5 (7.7%) | 0 (0%) | 0.42 |
| Percutaneous entry site | 30 (46.1%) | 2 (25.0%) | 0.42 |
| Gastrointestinal | 17 (26.1%) | 4 (50.0%) | 0.42 |
| Others | 13 (20%) | 2 (25.0%) | 0.42 |
| Blood transfusion | 28 (0.9%) | 3 (1.9%) | 0.76 |
| Postprocedure stroke and myocardial infarction | |||
| Postprocedure stroke | 5 (0.2%) | 1 (0.6%) | 0.18 |
| Periprocedural myocardial infarction | 45 (1.4%) | 4 (2.5%) | 0.29 |
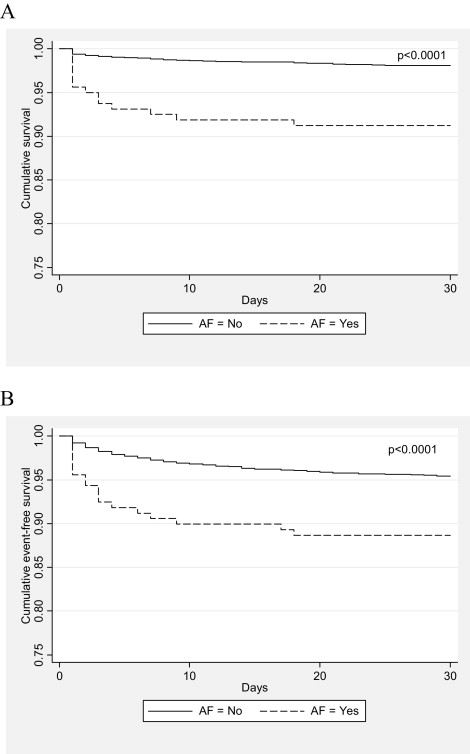
Thirty-day mortality was fourfold higher in patients with AF (9.9% vs 2.2%, p <0.0001) owing to a larger percentage of cardiovascular deaths (8.0% vs 1.7%, p <0.0001). The AF cohort also had excess overall major adverse cardiac events (p <0.001) and readmissions (p = 0.01) at 30 days ( Table 4 ). Cumulative event-free survival curves between the AF and SR groups for 30-day mortality and major adverse cardiac events are shown in Figure 1 . Postprocedure stroke and periprocedural MI were similar between the AF and SR groups. Of patients who had an MI or died within the first 30 days, 19% of these events were adjudicated to have been related to a definite or probable stent thrombosis. However, the incidence of definite or probable early stent thrombosis between patients with AF and those with SR was not different (0.6% vs 0.9%, p = NS).
| SR | AF | p Value | |
|---|---|---|---|
| (n = 3,145) | (n = 162) | ||
| Mortality | 68 (2.2%) | 16 (9.9%) | <0.001 |
| Cardiac death | 55 (1.7%) | 13 (8.0%) | <0.0001 |
| Myocardial infarction | 72 (2.3%) | 6 (3.7%) | 0.25 |
| Target lesion revascularization | 53 (1.7%) | 2 (1.2%) | 0.66 |
| Target vessel revascularization | 70 (2.2%) | 2 (1.2%) | 0.40 |
| Readmissions | 344 (11.1%) | 26 (17.8%) | 0.01 |
| Major adverse cardiac events | 174 (5.5%) | 21 (13%) | <0.001 |
The following significant univariate predictors were entered into multivariate binary logistic regression analyses for 30-day mortality and major adverse cardiac events: AF, drug-eluting stent use, age, diabetes, dyslipidemia, renal failure, cerebrovascular disease, peripheral arterial disease, heart failure, STEMI, glycoprotein IIb/IIIa inhibitor use, left main and left anterior descending coronary artery PCIs, complex lesion structure (American Heart Association/American College of Cardiology type B2/C), stent length, and in-hospital bleeding. AF was an independent predictor of 30-day mortality (odds ratio [OR] 2.78, 95% confidence interval [CI] 1.35 to 5.72, p = 0.01). Other multivariate predictors of 30-day mortality included STEMI as the indication for PCI (OR 8.86, 95% CI 4.45 to 17.64, p <0.001), renal failure (OR 5.53, 95% CI 2.64 to 11.57, p <0.001), peripheral arterial disease (OR 2.51, 95% CI 1.18 to 5.35, p = 0.02), and left main coronary artery PCI (OR 9.57, 95% CI 3.26 to 28.07, p <0.001) or left anterior descending coronary artery PCI (OR 2.16, 95% CI 1.30 to 3.58, p = 0.01).
At 30 days, independent multivariate predictors of major adverse cardiac events included STEMI (OR 2.10, 95% CI 1.43 to 3.06, p <0.001), renal failure (OR 2.00, 95% CI 1.17 to 3.45, p = 0.01), complex lesions (OR 1.76, 95% CI 1.23 to 2.53, p = 0.002), cerebrovascular disease (OR 1.70, 95% CI 1.03 to 2.83, p = 0.04), diabetes (OR 1.53, 95% CI 1.08 to 2.16, p = 0.02), left main coronary artery PCI (OR 4.23, 95% CI 1.83 to 9.76, p = 0.001) or left anterior descending coronary artery PCI (OR 1.48, 95% CI 1.09 to 2.02, p = 0.01), and in-hospital bleeding (OR 2.04, 95% CI 1.02 to 4.10, p = 0.04). However, AF was not an independent predictor of 30-day major adverse cardiac events (OR 1.66, 95% CI 0.96 to 2.88, p = 0.07), and drug-eluting stent use appeared protective (OR 0.56, 95% CI 0.38 to 0.82, p = 0.003).
The antithrombotic strategy captured at 30 days varied significantly between groups ( Table 5 ). Use of dual antiplatelet therapy was significantly lower in patients with AF compared to the SR group (86.3% vs 94.3%, p <0.001). More patients with AF were prescribed an oral anticoagulant alone, an oral anticoagulant with a single antiplatelet agent (aspirin or clopidogrel), or triple therapy (p <0.001 for all comparisons).



