Many patients with ST-elevation myocardial infarctions (STEMIs) have non–chest pain complaints and are given low priority during triage. This prospective, multicenter, observational, registry-based study investigated the impact of non–chest pain complaints on door-to-balloon (DTB) time and clinical outcomes. Patients with STEMI who had undergone primary percutaneous coronary intervention were compared with respect to the presence of chest pain or non–chest pain complaints as presenting symptoms. To eliminate biased estimates, a propensity score model was built, and 2 cohorts of 1:1 matched patients were obtained. Propensity matching identified 2 cohorts of 976 patients each. After comparing patients with chest pain and those with non–chest pain complaints, significant delays in the median DTB time were noted (74 vs 84 minutes, respectively; p <0.001). Non–chest pain complaints were independent predictors of DTB time in the multivariate linear regression models. In-hospital mortality (adjusted hazard ratio [HR] 1.402, 95% confidence interval [CI] 0.727 to 2.705, p = 0.313), all-cause mortality (adjusted HR 1.175, 95% CI 0.453 to 3.853, p = 0.642), and major adverse cardiac events at follow-up (adjusted HR 0.139, 95% CI 0.876 to 1.48, p = 0.331) did not differ between the 2 groups of patients. In conclusion, short- and long-term clinical outcomes in patients with STEMI with non–chest pain complaints do not differ from those of patients with chest pain as the presenting symptom, despite having delayed diagnosis and reperfusion.
Time to treatment is an essential predictive indicator of morbidity and mortality in patients with acute ST-elevation myocardial infarction (STEMI) receiving either pharmacologic or mechanical reperfusion therapy. In particular, the time to primary percutaneous coronary intervention (PCI) is strongly associated with mortality risk and is important regardless of the time from symptom onset to presentation or the patient’s baseline mortality risk. The independent predictors of delayed door-to-balloon (DTB) time include hospital transfer, nondaytime presentation, presentation at a low-volume center, and non–chest pain complaints as a presenting symptom. Many patients with STEMIs have non–chest pain complaints and tend to be given a low priority score during triage in the emergency department (ED). Thus, such patients might have a delayed diagnosis and may receive reperfusion at late stage after STEMI, resulting in prolonged DTB time and poor short-term clinical outcomes. However, the long-term clinical outcomes of such patients are not well studied. Accordingly, this study assessed the independent effects of non–chest pain complaints as presenting symptoms on DTB time and clinical outcomes in real-world practice.
Methods
In this prospective, multicenter, observational registry-based study, the data for 31,149 patients with STEMIs and non-STEMIs from 53 hospitals were retrieved from the Korean Acute Myocardial Infarction Registry, from 2006 to 2007, and from the Korea Working Group of Myocardial Infarction registry, from 2008 to 2012. The participating centers included university or community hospitals with facilities for PCI and on-site cardiac surgery. Data were collected by trained study coordinators using a standardized case report form and protocol. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in the study approval by the human research committee of each participating institution. Written informed consent for use of the data was also obtained from each patient.
The inclusion criteria for the present study were patients (1) ≥18 years old, (2) having ST-segment elevations >1 mm in at least 2 contiguous leads or presumably new left bundle branch block with elevated cardiac enzymes (troponin or myocardial band fraction of creatine kinase), and (3) undergoing primary PCI. Patients were excluded if they had missing DTB time information, or had PCI delayed for >12 hours after arrival ( Figure 1 ).
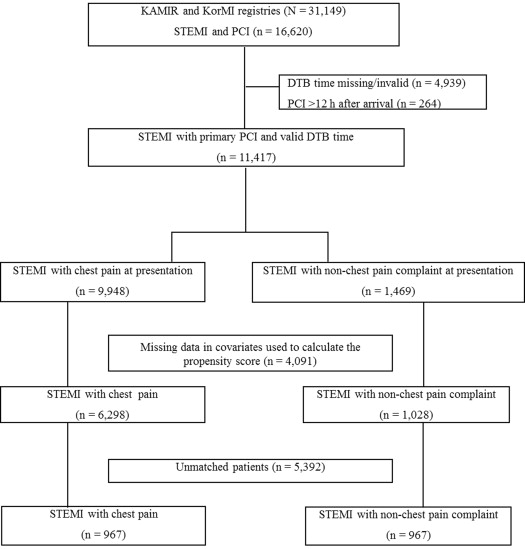
When a patient with chest pain, indicative of an acute STEMI, arrives at the ED, a fellow or third-grade resident in the cardiology department usually makes a decision regarding performing emergency angiography and PCI. Each patient received aspirin (200 to 300 mg) on admission, followed by daily aspirin (≥100 mg/day) for an indefinite period. Clopidogrel (300 to 600 mg) was also immediately administered, followed by daily doses (75 mg/day), thereafter. Cilostazol (200 mg/day) was administered, as needed. Angiotensin converting enzyme inhibitor or angiotensin receptor blocker, platelet glycoprotein IIb/IIIa inhibitor (i.e., abciximab or tirofiban), statins, unfractionated or low-molecular weight heparin, and β blockers were administered according to the attending doctor’s decision. All patients underwent emergency coronary angiography, with or without PCI according to the type of coronary lesion.
The primary outcomes were in-hospital mortality, all-cause death, and major adverse cardiac events (MACE, defined as all-cause death, recurrent myocardial infarction [MI], and any revascularization, including repeat-PCI and coronary artery bypass graft surgery) during follow-up. The secondary outcomes were DTB time and the proportions of patients with DTB time <90 and <60 minutes. Door-to-laboratory arrival (DTL) time, laboratory arrival-to-balloon (LTB) time, and symptom onset-to-door time were also assessed.
Non–chest pain complaints were defined as any symptoms other than substernal chest pain or discomfort with an ischemic characteristic, at presentation. All-cause death was defined as any death during or after the procedure and was considered to be of cardiac origin, unless a definite noncardiac cause could be established. Recurrent MI was defined as recurrent symptoms with new electrocardiographic changes compatible with MI or cardiac markers at least twice the upper limit of normal. Any revascularization was defined as a revascularization involving either the target or nontarget vessels. DTB, DTL, and LTB times were defined as the intervals between arrival at the hospital and intracoronary balloon inflation, arrival at the hospital and arrival at the laboratory, and arrival at the laboratory and intracoronary balloon inflation, respectively. Procedural success was defined as the Thrombolysis In Myocardial Infarction (TIMI) flow grade of 2 or 3 in the infarct-related artery and a residual stenosis of <20% in the treated segment at the end of the procedure.
Categorical variables are presented as numbers and percentages and were analyzed using Pearson’s chi-square test or Fisher’s exact test, as appropriate. Continuous variables are presented as means ± SD or median and interquartile range and were compared using the Student independent t test or the Mann-Whitney U test, as appropriate. A propensity score model was built to eliminate covariate differences; 2 cohorts of 1:1 nearest-neighbor–matched (i.e., a 1:1 matching algorithm without replacement, caliper width of 0.1) patients were consequently obtained. The propensity score was calculated using logistic regression covariates including age, gender, body mass index, past medical history (i.e., presence of ischemic heart disease, hypertension, diabetes, dyslipidemia, and smoking status), Killip class, resuscitation, initial electrocardiographic findings, left ventricular ejection fraction, initial laboratory data (i.e., serum creatinine, glucose, troponin I, low-density and high-density lipoprotein cholesterols, and prohormone brain natriuretic peptide), interhospital transfer, ambulance usage, and angiographic characteristics (i.e., number of diseased vessels, infarct-related artery, preprocedural TIMI flow grade 0 or 1, postprocedural TIMI flow grade 2 or 3, lesion type B2 or C, and use of drug-eluting stents). After propensity matching, all absolute standardized differences were <10%, indicating adequate matching.
In-hospital mortality and clinical outcomes during follow-up were analyzed using the Kaplan-Meier method. Intergroup comparisons were made using the log-rank test. Cox proportional hazard regression models, adjusted for propensity scores, were used to determine the rates of all-cause death, cardiac death, revascularization, recurrent MI, and the composite of MACEs during follow-up in both patient groups.
Multiple linear regression models were constructed to determine the effect of non–chest pain complaints on DTB time, after adjusting for clinical covariates. Because the DTB time data were nonlinear, they were log-transformed before analysis. The DTB model was sequentially constructed and included the following variables in blocks: age, gender, cardiovascular risk factors (i.e., dyslipidemia, hypertension, diabetes, family history of heart disease, history of smoking, and previous coronary artery disease), infarction characteristics (i.e., ST-segment elevation and infarct location on the electrocardiogram [ECG]), hemodynamics (i.e., heart rate, systolic blood pressure, Killip class III or IV, and resuscitation), logistics (i.e., treatment method at arrival and transfer from other hospital), and angiographic characteristics (i.e., triple-vessel disease, type B2 or C lesion, and preprocedural TIMI flow grade 0 or 1 and postprocedural TIMI flow grade 2 or 3).
Complete, available data sets were used for all analyses; missing continuous variables were imputed using the expectation-maximization technique. The level of statistical significance was set at p <0.05. All analyses were performed using SPSS 21.0 for Windows (SPSS, Chicago, Illinois) and R version 2.14.2, a freely distributed statistical package.
Results
Non–chest pain complaints were reported by1028 of 7326 (14%) patients before propensity score matching. The patients’ baseline clinical and angiographic characteristics are listed in Table 1 . There were significant differences in baseline and angiographic characteristics between groups, which disappeared after matching with all absolute standardized differences <10%, suggesting adequate matching. In the propensity-matched cohort, anterior wall infarctions were observed in the initial ECGs of 58.4% and 59.1% of the patients with chest pain and non–chest pain complaints, respectively. Similarly, occlusion of the left anterior descending artery was observed, angiographically, in 51% and 51.5% of the patients, respectively. Procedural success was 98.8% (chest pain) and 98.7% (non–chest pain) in the 2 groups, with similar rates of postprocedural TIMI flow grade 2 or 3.
| Variables | Before propensity score-matching | After propensity score-matching | ||||
|---|---|---|---|---|---|---|
| Chest pain (n = 6298) | Non-chest pain complaints (n = 1028) | p | Chest pain (n = 976) | Non-chest pain complaints (n = 976) | p | |
| Age (y± SD) | 61.8 ± 12.6 | 64.9 ± 12.9 | <0.001 | 64.4 ± 12.3 | 64.8 ± 13 | 0.486 |
| Males | 4729 (75.1%) | 723 (70.3%) | 0.001 | 703 (72%) | 692 (70.9%) | 0.581 |
| BMI (kg/m 2 ± SD) | 24 ± 3.2 | 23.8 ± 3.1 | 0.077 | 23.9 ± 3.4 | 23.9 ± 3.2 | 0.748 |
| Hypertension | 2815 (44.7%) | 510 (49.6%) | 0.003 | 469 (48.1%) | 480 (49.2%) | 0.618 |
| Diabetes mellitus | 1451 (23%) | 291 (28.3%) | <0.001 | 266 (27.3%) | 278 (28.5%) | 0.545 |
| Current smoking | 2317 (36.8%) | 446 (43.4%) | <0.001 | 409 (41.9%) | 412 (42.2%) | 0.989 |
| Dyslipidemia ∗ | 696 (11.1%) | 70 (6.8%) | <0.001 | 77 (7.9%) | 67 (6.9%) | 0.387 |
| Previous IHD † | 714 (11.3%) | 85 (8.3%) | 0.003 | 84 (8.6%) | 78 (8%) | 0.623 |
| Family history of IHD | 480 (7.6%) | 43 (4.2%) | <0.001 | 54 (5.5%) | 41 (4.2%) | 0.382 |
| Systolic BP (mmHg± SD) | 126 ± 28 | 124 ± 27 | 0.001 | 126 ± 28 | 124 ± 26 | 0.097 |
| Heart rate (beats/min± SD) | 76 ± 18 | 77 ± 20 | 0.074 | 76 ± 19 | 76 ± 19 | 0.491 |
| Killip class, III/IV | 628 (10%) | 164 (16%) | <0.001 | 137 (14%) | 144 (14.8%) | 0.652 |
| Resuscitation at arrival | 85 (1.3%) | 60 (5.8%) | <0.001 | 43 (4.4%) | 53 (5.4%) | 0.295 |
| Anterior wall infarct on ECG | 3408 (54.1%) | 602 (58.6%) | 0.008 | 570 (58.4%) | 577 (59.1%) | 0.748 |
| LBBB on ECG | 283 (4.5%) | 53 (5.2%) | 0.347 | 50 (5.1%) | 51 (5.2%) | 0.919 |
| LVEF (%± SD) | 51.2 ± 11.2 | 52 ± 11.8 | 0.04 | 52.2 ± 11.6 | 52 ± 11.8 | 0.73 |
| Pro-BNP (pg/dL± SD) | 1411 ± 3949 | 2884 ± 5959 | <0.001 | 2397 ± 7051 | 2629 ± 5299 | 0.401 |
| Peak troponin I (ng/mL± SD) | 61.8 ± 119.1 | 62.3 ± 75 | 0.916 | 65.2 ± 78.8 | 62.4 ± 73.3 | 0.425 |
| Creatinine (mg/dL± SD) | 1.09 ± 1.19 | 1.17 ± 1.18 | 0.044 | 1.14 ± 2.26 | 1.16 ± 1.19 | 0.773 |
| Glucose (mg/dL± SD) | 172.7 ± 74.6 | 182.4 ± 90.2 | <0.001 | 180.6 ± 81.9 | 181.2 ± 88 | 0.877 |
| LDL-C (mg/dL± SD) | 116.9 ± 41.3 | 114.9 ± 37.4 | 0.142 | 115 ± 35.7 | 115.3 ± 36.6 | 0.879 |
| HDL-C (mg/dL± SD) | 44.5 ± 19.4 | 43.3 ± 11.4 | 0.058 | 42.9 ± 10.8 | 43.4 ± 11.4 | 0.424 |
| Inter-hospital transfer | 2630 (41.8%) | 276 (26.8%) | <0.001 | 254 (26%) | 254 (26%) | 1 |
| Use of ambulance | 1840 (29.2%) | 220 (21.4%) | <0.001 | 206 (21.1%) | 201 (20.6%) | 0.781 |
| Number of diseased vessels | ||||||
| 1 | 3115 (49.5%) | 519 (50.5%) | 0.002 | 504 (51.6%) | 496 (50.8%) | 0.852 |
| 2 | 1878 (29.8%) | 287 (27.9%) | 278 (28.5%) | 272 (27.9%) | ||
| 3 | 1203 (19.1%) | 188 (18.3%) | 167 (17.1%) | 176 (18%) | ||
| Left main involvement | 102 (1.6%) | 34 (3.3%) | 27 (2.8%) | 32 (3.3%) | ||
| Infarct-related coronary artery | ||||||
| Left main | 61 (1%) | 13 (1.3%) | 0.368 | 11 (1.1%) | 12 (1.2%) | 0.99 |
| Left anterior descending | 3280 52.1%) | 526 (51.2%) | 498 (51%) | 503 (51.5%) | ||
| Left circumflex | 582 (9.2%) | 89 (8.7%) | 85 (8.7%) | 83 (8.5%) | ||
| Right | 2372 (37.7%) | 398 (38.7%) | 379 (38.8%) | 376 (38.5%) | ||
| Pre-procedural TIMI flow 0/1 | 4455 (70.7%) | 659 (64.1%) | <0.001 | 612 (62.7%) | 623 (63.8%) | 0.898 |
| Post-procedural TIMI flow 2/3 | 6170 (98%) | 1012 (98.4%) | 0.308 | 964 (98.8%) | 964 (98.7%) | 0.999 |
| Lesion Type B2/C | 5013 (79.6%) | 876 (85.2%) | <0.001 | 828 (84.9%) | 830 (85.1%) | 0.951 |
| Use of drug-eluting stents | 5367 (85.2%) | 791 (76.9%) | <0.001 | 762 (78.1%) | 753 (77.2%) | 0.751 |
∗ Dyslipidemia is defined as a prior diagnosis of dyslipidemia or statin treatment.
† previous IHD is defined as a prior history of angina, percutaneous coronary intervention, myocardial infarction, and/or coronary artery bypass graft surgery.
Compared with patients with chest pain, significantly more patients with non–chest pain complaints had delayed medical contact (2.99 vs 4.25 hours, p = 0.003; Table 2 ). Compared with patients with chest pain, those with non–chest pain complaints also had significantly prolonged DTL time (53 vs 60 minutes, p <0.001), LTB time (20 vs 22 minutes, p <0.001), and DTB time (74 vs 84 minutes, p <0.001). A significantly smaller proportion of patients with non–chest pain complaints, compared with those with chest pain, underwent primary PCI, with a DTB time <90 minutes (67.9 vs 54.9%, respectively; p <0.001) and a DTB time <60 minutes (28.2 vs 20.2%, respectively; p <0.001). These factors were similar in the entire cohort before propensity score matching.
| Before propensity score-matching | After propensity score-matching | |||||
|---|---|---|---|---|---|---|
| Chest pain (n = 6298) | Non-chest pain complaints (n = 1028) | p | Chest pain (n = 976) | Non-chest pain complaints (n = 976) | p | |
| Symptom onset-to-door time (h) | 2.83 (1.41, 5.91) | 4.21 (2.15, 10.07) | 0.011 | 2.99 (1.73, 5.6) | 4.25 (2.16, 10.29) | 0.003 |
| Symptom onset-to-balloon time (h) | 4.59 (2.88, 8.5) | 6.45 (3.75, 12.88) | 0.005 | 4.54 (3.03, 7.82) | 6.5 (3.75, 13.05) | 0.001 |
| Door-to-laboratory arrival time (min) | 55 (37, 90) | 60 (40, 110) | <0.001 | 53 (36, 77) | 60 (40, 110) | <0.001 |
| Laboratory arrival-to-balloon (min) | 20 (15, 28) | 22 (17, 30) | <0.001 | 20 (15, 27) | 22 (17, 30) | <0.001 |
| Door-to-balloon time (min) | 77 (57, 115) | 85 (63, 139) | <0.001 | 74 (56, 103) | 84 (63, 139) | <0.001 |
| Patients with DTB time <90 min | 3921 (62.3%) | 562 (54.7%) | <0.001 | 663 (67.9%) | 536 (54.9%) | <0.001 |
| Patients with DTB time <60 min | 1755 (27.9%) | 212 (20.6%) | <0.001 | 275 (28.2%) | 197 (20.2%) | <0.001 |
Multivariate linear regression analysis, adjusted for clinical confounders, was performed to assess the effect of non–chest pain complaints on DTB time in the propensity score-matched cohort. Unadjusted and adjusted associations between the non–chest pain complaints group and DTB time are summarized in Table 3 ; the determinants of DTB time in the multivariate regression analysis are listed in Table 4 . In the final model, non–chest pain complaints remained a significant predictor of delayed DTB time, even after adjusting for important clinical covariates.
| Estimated ( β ) unstandardized coefficient | p | 95% CI | |
|---|---|---|---|
| Unadjusted | |||
| non-chest pain complaint | 0.136 | <0.001 | 0.120–0.235 |
| Adjusted model 1 | |||
| non-chest pain complaint | 0.137 | <0.001 | 0.121–0.236 |
| Adjusted model 2 | |||
| non-chest pain complaint | 0.137 | <0.001 | 0.121–0.236 |
| Adjusted model 3 | |||
| non-chest pain complaint | 0.136 | <0.001 | 0.121–0.234 |
| Adjusted model 4 | |||
| non-chest pain complaint | 0.139 | <0.001 | 0.125–0.238 |
| Adjusted model 5 | |||
| non-chest pain complaint | 0.139 | <0.001 | 0.124–0.237 |
| Adjusted model 6 | |||
| non-chest pain complaint | 0.141 | <0.001 | 0.128–0.239 |
| Estimate ( β ) unstandardized coefficient | p | 95% CI | |
|---|---|---|---|
| Non-chest pain complaints | 0.141 | <0.001 | 0.128–0.239 |
| LBBB on ECG | 0.133 | <0.001 | 0.266–0.519 |
| Systolic blood pressure | 0.058 | 0.014 | 0.0001–0.003 |
| Killip class III/IV | 0.048 | 0.045 | 0.002–0.176 |
In the propensity-matched cohort, 228 (11.7%) patients experienced at least 1 primary outcome (i.e., all-cause death, recurrent MI, and any revascularization) during a 2-year follow-up (median 358 days, interquartile range 49 to 426 days). The rates of in-hospital mortality and cardiac death during hospitalization did not differ significantly between the chest pain and non–chest pain complaints groups ( Table 5 ). Similarly, the Kaplan-Meier estimates for survival free from in-hospital deaths did not differ between the groups ( Figure 2 ). The rates of complications (chest pain vs non–chest pain complaints groups), including ventricular tachycardia and/or fibrillation (1.6 vs 1.9%, p = 0.743), heart failure (0 vs 0.1%, p = 0.513), cardiogenic shock (1.3 vs 2.9%, p = 0.051), cerebrovascular attack (0.4 vs 0.4%, p = 0.847), renal failure (0.2 vs 0.3%, p = 0.766), multiorgan failure (0 vs 0.1%, p = 0.513), and bleeding (0 vs 0.5%, p = 0.069) did not differ significantly between groups during hospitalization. Clinical outcomes and cumulative survival during the follow-up period are listed in Table 5 and Figure 3 . All-cause death (chest pain vs non–chest pain complaints groups; 4.6 vs 5.9%, adjusted HR 1.175, p = 0.642), cardiac death, recurrent MI, any revascularization, and the composite of MACE (10.2 vs 13.2%, adjusted HR, 1.139, p = 0.331) did not differ significantly between the 2 groups. In the entire cohort, before propensity matching, the rates of in-hospital cardiac deaths were not different between the chest pain and non–chest pain complaints groups, with in-hospital mortality showing a marginally significant difference (2.2 vs 4.5%, respectively, adjusted HR 1.94, p = 0.07). All-cause death rates during follow-up differed significantly between chest pain and non–chest pain complaints groups (4% vs 8%, adjusted HR 1.559, p = 0.027), with the rates of cardiac death, recurrent MI, any revascularization, and composite MACE not showing any differences between the groups ( Supplementary Table 1 ).



