A periprocedural myocardial infarction, defined as the advent of new Q-waves or a creatine kinase-MB elevation >8× normal has been previously validated as predictive of subsequent mortality. We examined the effects of using this clinically relevant definition of periprocedural myocardial infarction instead of the original protocol definition on outcomes in the recent PROTECT II [A Prospective, Multi-center, Randomized Controlled Trial of the IMPELLA RECOVER LP 2.5 System Versus Intra Aortic Balloon Pump (IABP) in Patients Undergoing Non Emergent High Risk PCI] trial. In this trial, patients who were undergoing high-risk percutaneous coronary intervention (PCI) were randomized to either an intra-aortic balloon pump (IABP, n = 211) or a left ventricular assist device (Impella, n = 216). All eligible patients per study protocol were included in the analysis. Patient outcomes were compared up to 90 days, the longest available follow-up, on the composite end points of major adverse events (MAE) and major adverse cardiac and cerebral events (MACCE = death, stroke, myocardial infarction, and repeat revascularization). At 90 days, the rates of both composite end points were lower in the Impella group compared with the IABP group (MAE, 37% vs 49%, p = 0.014 respectively; MACCE, 22% vs 31%, p = 0.034 respectively). There were no differences in death or large myocardial infarction between the 2 arms. By multivariable analysis, treatment with Impella as opposed to IABP was an independent predictor for freedom from MAE (odds ratio = 0.75 [95% confidence interval 0.61 to 0.92], p = 0.007) and MACCE (odds ratio = 0.76 [95% confidence interval 0.61 to 0.96], p = 0.020) at 90 days postprocedure. In conclusion, hemodynamic support with Impella compared with IABP during high-risk PCI in the PROTECT-II trial resulted in improved event-free survival at 3-month follow-up; this finding was further supported by multivariate analyses.
High-risk percutaneous coronary intervention (PCI) in patients with multivessel or unprotected left main coronary artery disease and severely depressed left ventricular function poses significant technical challenges and procedural risk. Additionally, this patient population typically possesses additional co-morbidities and distal coronary artery disease that often precludes the option of coronary artery bypass grafting (CABG). Hemodynamic support may be required during these high-risk procedures. We have previously reported the results of a randomized trial comparing outcomes for patients supported with Intra-aortic balloon pump (IABP) or Impella 2.5 left ventricular assist device (A Prospective, Multi-center, Randomized Controlled Trial of the IMPELLA RECOVER LP 2.5 System Versus Intra Aortic Balloon Pump (IABP) in Patients Undergoing Non Emergent High Risk PCI [PROTECT II]). At the time of the trial design, the just-published universal definition of myocardial infarction was used to define periprocedural myocardial infarction (pMI) with a cutoff of ≥3× the upper limit of normal (ULN) for cardiac biomarker elevation. However, numerous studies have documented the limitations of the universal definition and this threshold in the determination of outcome measures in clinical trials or as a quality metric in routine care. Small to moderate elevations of troponin or creatine kinase (CK)-MB biomarkers after PCI have a benign clinical course. However, less controversy exists as to whether large pMI are linked to adverse outcomes. Indeed, in the bare-metal-stent era, the largest studies demonstrated a prognostic relation with subsequent mortality only with new Q-wave infarction or CK-MB elevation >8 to 10× ULN was present More recently, large multicenter studies in the drug-eluting-stent era confirmed that at least a 10× elevation of CK-MB after PCI was required for a long-term effect on mortality to be seen. For this reason, we have reexamined the outcomes of PROTECT II using a prognostically relevant definition of myocardial infarction (MI) and broadened the strength of our analyses by including multivariable testing for predictors of cardiovascular adverse events.
Methods
PROTECT II was a prospective multicenter randomized trial conducted in 112 sites in the United States, Canada, and Europe, and its methods have been previously reported in detail. In brief, patients who underwent high-risk PCI requiring circulatory support and were randomized to the Impella 2.5 device versus IABP. Inclusion criteria were a nonemergent PCI on an unprotected left main or last patent coronary vessel with a left ventricular ejection fraction ≤35% or PCI in patients with 3-vessel disease and left ventricular ejection fraction ≤30%. Patients with recent MI and persistent elevation of cardiac enzymes were excluded. The study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Review Committee of each participating center, and all patients provided written informed consent before enrollment. U.S. Food and Drug Administration Investigational Device Exemption (IDE# G050017) was obtained by the study sponsor (Abiomed, Danvers, Massachusetts) in November 2007.
Randomization to either the Impella 2.5 (n = 225) or the IABP (n = 223) occurred in a 1:1 ratio with 2 levels of stratification: geographic region (n = 5) and angioplasty indication (unprotected left main/last remaining vessel vs 3-vessel disease). Crossover from 1 study arm to the other was not permitted. Operators were asked to aim for the most complete revascularization of the myocardium at jeopardy in a single procedure. Procedural anticoagulation with unfractionated heparin and an activated clotting time value >250 seconds was required before device implantation. All other procedural details regarding drug and device choices were left to the discretion of the operator. After PCI, if patients could be weaned from hemodynamic support, hemostasis was achieved by direct manual pressure when ACT <180 seconds or the “pre-close” technique as previously reported. Patients were then admitted to the coronary care unit for observation and discharged when appropriate. Follow-up was scheduled at 30 and 90 days postprocedure.
The complete set of definitions and endpoints of the PROTECT-II trial have been reported previously. The primary end point of the study was a composite of 10 individual adverse event components of different prognostic values ranging from death to angiographic failure. Periprocedural MI was recorded if CK-MB after PCI rose to ≥3× the local laboratory ULN. For the purpose of the present post hoc analysis, we sought to evaluate the study results with respect to more prognostically relevant composite endpoints, specifically major adverse cardiac and cerebrovascular events (MACCE), defined as all-cause mortality, myocardial infarction, stroke or transient ischemic attack (TIA), or any repeat revascularization procedure (PCI or CABG). For this purpose MI was defined as the development of new Q-waves or CK-MB elevation ≥8× ULN within 72 hours post-PCI for pMI, or as ≥2× ULN beyond 72 hours of the PCI for spontaneous MI (sMI), the latter representing the same criteria in the original PROTECT II protocol. When CK-MB was not available, troponin values were used with the same thresholds. Composite major adverse events (MAE) are defined as the occurrence of any MACCE or the need for a cardiac or vascular operation (including a vascular operation for limb ischemia); acute renal insufficiency; severe intraprocedural hypotension requiring therapy or cardiopulmonary resuscitation or ventricular tachycardia requiring cardioversion; aortic insufficiency; or angiographic failure of PCI.
Data collection, management, and monitoring; events adjudication; and statistical analysis were conducted by an independent academic clinical research organization (Harvard Clinical Research Institute, Boston, Massachusetts). Not-for-profit independent academic central core laboratories analyzed all angiographic films (Beth Israel Deaconess, Boston, Massachusetts) and echocardiographic data (Duke Clinical Research Institute, Durham, North Carolina) to determine angiographic success and device safety (including aortic insufficiency), respectively. An independent Clinical Events Committee adjudicated all MACCE and study end points blinded to treatment group assignment (Harvard Clinical Research Institute, Boston, Massachusetts).
As previously described, 452 patients were enrolled in the study, and the overall patient characteristics and primary study results have been previously published. The present analysis is performed on the intention-to-treat analysis (ITT) population (n = 448) and the per protocol (PP) study population (n = 427), which includes all consented randomized patients who met the protocol eligibility criteria (216 for Impella 2.5 and 211 for IABP). Outcomes are reported at 30 days and for the longest available follow-up of 90 days.
The data are expressed as mean ± SD, median (range), or proportions as appropriate. Univariable parametric analysis was performed using a 2-tailed unpaired t test or a nonparametric Mann-Whitney test for continuous outcomes. Pearson’s chi-square or Fisher’s exact tests were used as appropriate for nominal data. A 1-way analysis of variance was performed to compare baseline and procedural characteristics between patient groups that have different levels of cardiac biomarker release. Kaplan-Meier estimates of the cumulative incidence of MAE and MACCE were calculated, and a log-rank test was performed to compare the clinical outcomes between the 2 study arms. A stepwise multivariable logistic regression analysis with backward elimination was used to evaluate the independent predictors of MACCE and MAE. Multiple imputation technique was used to account for missing data in the explanatory variables of the logistic regression model. No imputations were performed for any component of the dependent variable of MAE or MACCE. All p values were 2-tailed and considered significant when the probability was <0.05. The statistical analyses for this report were performed by the Harvard Clinical Research Institute using Statistical Analysis System version 9.2 (SAS Institute, Cary, North Carolina).
Results
The baseline features and procedural outcomes of the ITT groups have been previously reported, and thus the PP data are shown here. Baseline characteristics in the per protocol population were similar between groups, apart from higher rates of congestive heart failure, current smokers, and previous aortocoronary bypass surgery and a trend toward a lower rate of chronic renal failure in the Impella arm ( Table 1 ). The overall patient population was anatomically complex and very high risk, with mean left ventricular ejection fraction 24%, and high mean risk stratification scores as follows: Society of Thoracic Surgery (STS) mortality score 6%, additive EuroSCORE 9%, logistic EuroSCORE 19%, STS combined mortality and morbidity score 30%, and Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery score 30. Two-thirds of patients were declared inoperable by onsite cardiac surgeons. Angiographic characteristics were similar between the groups except for a higher rate of subtotal occlusions in the Impella arm. Use of glycoprotein IIb/IIIa inhibitors was less frequent and rotational atherectomy use was more frequent and extensive in the Impella group compared with the IABP group. There was also more use of contrast media in the Impella arm compared with IABP.
| Variable | Impella 2.5 (n = 216 Patients) (n = 547 Lesions) | IABP (n = 211 Patients) (n = 530 Lesions) | p Value |
|---|---|---|---|
| Age (yrs) | 68 ± 11 | 67 ± 11 | 0.58 |
| Men | 81% | 82% | 0.70 |
| Current smoker | 51% | 39% | 0.04 |
| Previous congestive heart failure | 91% | 83% | 0.01 |
| Current CHF NYHA Class III/IV | 59% | 55% | 0.43 |
| Diabetes mellitus | 53% | 49% | 0.41 |
| Renal insufficiency | 23% | 30% | 0.09 |
| Peripheral vascular disease | 25% | 27% | 0.70 |
| Chronic obstructive pulmonary disease | 26% | 29% | 0.47 |
| Previous CABG | 39% | 29% | 0.02 |
| LVEF | 23 ± 6 | 24 ± 6 | 0.26 |
| Not a candidate for surgery | 63% | 65% | 0.83 |
| STS mortality score | 6 ± 6 | 6 ± 7 | 0.56 |
| SYNTAX score | 30 ± 13 | 30 ± 14 | 0.59 |
| Additive EuroSCORE | 9 ± 6 | 8 ± 4 | 0.22 |
| Logistic EuroSCORE | 19 ± 17 | 19 ± 18 | 0.95 |
| STS Morbidity/Mortality score | 29 ± 15 | 31 ± 16 | 0.17 |
| Left main target lesion location | 12% | 10% | 0.42 |
| Lesion length >20 (mm) | 17% | 15% | 0.40 |
| Thrombus | 1.1% | 0.8% | 0.55 |
| Calcification moderate or severe | 39% | 36% | 0.30 |
| Ulceration | 5.3% | 6.2% | 0.52 |
| Aneurysm | 4.2% | 5.1% | 0.50 |
| Preprocedural TIMI flow grade | |||
| 0 | 3.1% | 4.3% | 0.29 |
| 1 | 3.9% | 1.3% | 0.009 |
| 2 | 5.0% | 3.8% | 0.34 |
| 3 | 88.1% | 90.6% | 0.19 |
| Number of lesions attempted | 2.9 ± 1.5 | 2.9 ± 1.5 | 0.98 |
| Number of stents placed | 3.1 ± 1.8 | 3.0 ± 1.9 | 0.30 |
| Use of heparin | 94% | 82% | <0.001 |
| Glycoprotein IIb/IIIa inhibitors | 13% | 26% | <0.001 |
| Total contrast media (ml) | 267 ± 141 | 241 ± 115 | 0.04 |
| Rotational atherectomy | 15% | 10% | 0.09 |
| Median no. of passes/lesion (IQR) | 3 (2–5) | 1 (1–2) | 0.001 |
| Median no. of passes/patient (IQR) | 5.0 (3.5–9.5) | 2.0 (2.0–4.0) | 0.003 |
| Median RA time/lesion (IQR), s | 60 (40–118) | 40 (20–47) | 0.004 |
| RA of left main artery | 8% | 3% | 0.02 |
| Saphenous vein graft treatment | 13% | 10% | 0.32 |
| Vein graft PCI or native artery Rotablator | 26% | 19% | 0.05 |
| Total support time, h | 1.9 ± 2.7 | 8.2 ± 21.1 | <0.001 |
| Discharge from cath lab on device | 6% | 38% | <0.001 |
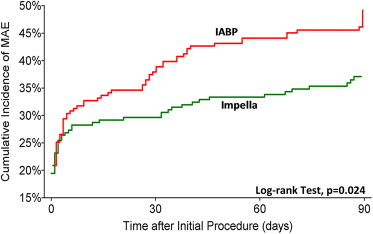
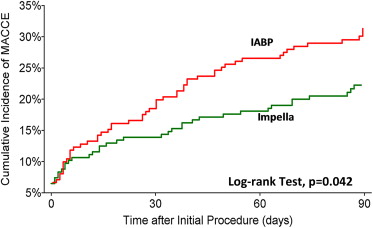
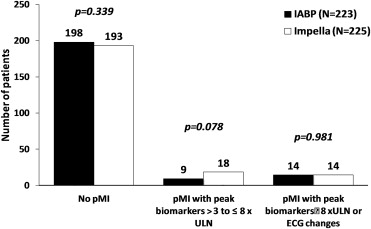
Outcome measures at 30 and 90 days are reported in Table 2 and Figures 1 and 2 . Fewer MAE and MACCE were observed in the Impella arm compared with the IABP arm, with concordance between the ITT and PP analyses. The rates of periprocedural MI did not significantly differ in any comparison performed. There was equivalence between treatment arms for elevations ≥8× ULN ( Figure 3 ). In contrast, numerically more patients in the Impella group had biomarker elevations between 3 and 8× ULN.
| Variable | PP Treated Population | ITT Population | ||||
|---|---|---|---|---|---|---|
| Impella 2.5 (n = 216) | IABP (n = 211) | p Value | Impella 2.5 (n = 224) | IABP (n = 219) | p Value | |
| 30-day event rates | ||||||
| MAE | 30% | 40% | 0.04 | 31% | 38% | 0.11 |
| MACCE | 14% | 20% | 0.10 | 15% | 19% | 0.23 |
| Death/MI | 12.5% | 13.3% | 0.81 | 13.3% | 12.6% | 0.82 |
| Death/stroke/MI | 12.5% | 15.2% | 0.43 | 13.3% | 14.4% | 0.74 |
| Death | 6.9% | 6.2% | 0.74 | 7.6% | 5.9% | 0.47 |
| Stroke/TIA | 0.0% | 1.9% | 0.04 | 0.0% | 1.8% | 0.04 |
| Myocardial infarction | 5.6% | 7.1% | 0.51 | 5.8% | 6.8% | 0.67 |
| Periprocedural MI (>8× ULN or ECG) | 3.7% | 6.2% | 0.24 | 3.6% | 5.9% | 0.25 |
| Spontaneous MI | 1.9% | 0.9% | 0.43 | 2.2% | 0.9% | 0.26 |
| Repeat revascularization | 1.4% | 4.7% | 0.04 | 1.3% | 4.5% | 0.05 |
| 90-day event rates | ||||||
| MAE | 37% | 49% | 0.01 | 37% | 47% | 0.03 |
| MACCE | 22% | 31% | 0.03 | 22% | 30% | 0.06 |
| Death/MI | 17.2% | 20.0% | 0.46 | 17.9% | 19.2% | 0.72 |
| Death/stroke/MI | 18.1% | 22.4% | 0.28 | 18.8% | 21.9% | 0.41 |
| Death | 11.6% | 9.0% | 0.38 | 12.1% | 8.7% | 0.24 |
| Stroke/TIA | 0.0% | 2.4% | 0.24 | 0.9% | 2.7% | 0.14 |
| Myocardial infarction | 5.6% | 11.0% | 0.04 | 5.8% | 10.5% | 0.07 |
| Periprocedural MI (>8× ULN or ECG) | 3.7% | 6.2% | 0.24 | 3.6% | 5.9% | 0.24 |
| Spontaneous MI | 1.9% | 4.8% | 0.09 | 2.2% | 4.6% | 0.17 |
| Repeat revascularization | 3.7% | 8.6% | 0.04 | 3.6% | 8.2% | 0.04 |

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


