Premature antiplatelet therapy discontinuation (ATD) after drug-eluting stent (DES) implantation is known to predict stent thrombosis (ST). However, recent data suggest that a shorter antiplatelet therapy duration is safe with newer generation DESs. The study aimed to compare the impact of early and late clopidogrel ATDs on ST in a real-world registry of first- and second-generation DES use. A total of 6,236 patients who underwent DES implantation were analyzed retrospectively: 4,217 received first-generation DESs (sirolimus- and paclitaxel-eluting stents) and 2,019 received second-generation DESs (everolimus-eluting stents). Within each DES cohort, patients were categorized into timing of clopidogrel discontinuation within 1 year: early (<3 months), late (3 to 12 months), and continued. ST rates and clinical outcomes at 1 year were analyzed. There were 341 patients (8.1%) in the first-generation DES group and 126 patients (6.2%) in the second-generation DES group who discontinued clopidogrel within the first year. Definite and probable ST rates were 3.8% for early ATD, 2.5% for late ATD, and 0.5% for continued (p = 0.001) in the first-generation DES cohort, whereas there were no definite or probable ST events in early and late ATDs and 0.5% for continued in the second-generation DES cohort. Major adverse cardiac event rates were 9.9% for early ATD, 5.6% for late ATD, and 0.9% for continued (p <0.001) in the first-generation DES cohort and 5.5% for early ATD, 7.4% for late ATD, and 1.5% for continued (p <0.001) in the second-generation DES cohort. In conclusion, ATD within the first year is associated with increased ST events with first-generation DESs, whereas ATD appears safe with second-generation DESs with regard to ST. However, ATD is associated with greater mortality and major adverse cardiac events in both first- and second-generation DESs. Thus, this study supports ATD if required based on physician discretion with the use of second-generation DESs but cannot rule out potential benefit for longer duration of dual antiplatelet therapy even when second-generation DESs are used.
In the last decade, percutaneous coronary intervention (PCI) with drug-eluting stents (DESs) has largely replaced bare-metal stent use because of their superior efficacy in preventing restenosis. From earlier reports, however, there were safety concerns with DESs regarding the increased risks of late and very late stent thromboses (STs) compared with bare-metal stents. These late thrombotic events were hypothesized to be due to delayed vessel healing, and the focus on dual antiplatelet therapy of aspirin and a thienopyridine has grown in parallel with concerns regarding ST. Such concerns, together with earlier studies in bare-metal stents, have led to current guideline recommendations of prolonged dual antiplatelet therapy of at least 1 year after DES implantation among the American societies and 6 to 12 months in Europe. Concurrently, observational studies have identified premature clopidogrel discontinuation to be a strong predictor of ST after DES implantation. These studies, however, evaluated outcomes using first-generation DESs. Newer generation DESs have since emerged and are proved to be equal or superior in antirestenotic effect, while demonstrating a consistently better safety profile with lower ST rates compared with first-generation DESs.
Moreover, data from recent trials suggest that perhaps a shorter duration of dual antiplatelet therapy is safe in the newer generation DESs. Certain second-generation stents such as Xience (Abbott Vascular, Santa Clara, California) and Resolute (Medtronic, Santa Rosa, California) have recently received indication in Europe allowing interruption of dual antiplatelet therapy beyond 1 to 3 months. However, the safety of short-term dual antiplatelet therapy is still not clearly defined in the real world. We aimed to compare the impact of clopidogrel discontinuation within the first year of stent implantation in patients receiving first- and second-generation DESs. Specifically, we aimed to describe the effect of early versus late antiplatelet therapy discontinuation (ATD) on ST in first- and second-generation DES use in a real-world clinical registry.
Methods
The study population included 6,236 consecutive patients who underwent PCI with DESs from 2003 to 2012 at MedStar Washington Hospital Center (Washington, DC), with available 1-year follow-up information. Two separate DES generation cohorts were analyzed. The first-generation DES cohort (n = 4,217) consisted of patients who received sirolimus-eluting stents (Cypher; Cordis, Johnson and Johnson, Miami Lakes, Florida) and paclitaxel-eluting stents (Taxus; Boston Scientific Corp, Natick, Massachusetts). The second-generation DES cohort (n = 2,019) consisted of patients who received everolimus-eluting stents (Xience V; Abbott Vascular, Santa Clara, California or Promus; Boston Scientific Corp, Natick, Massachusetts). Within each DES generation cohort, patients were categorized by their timing of clopidogrel discontinuation: early ATD (<3 months from DES implantation), late ATD (3 to 12 months from DES implantation), and continued (up to 12 months). All patients provided written informed consent, and the study complied with the Declaration of Helsinki. The institutional review boards from MedStar Washington Hospital Center and MedStar Health Research Institute (Washington, DC) approved the present study.
PCI was performed according to guidelines present at the time of procedure. All patients received aspirin 325 mg and clopidogrel 300 to 600 mg periprocedurally. The anticoagulation regimen was chosen at the operator’s discretion and included unfractionated heparin adjusted to a targeted activated clotting time or bivalirudin 0.75 mg/kg followed by an infusion of 1.75 mg/kg/hour for the duration of the procedure. The interventional strategy, together with the use of adjunct pharmacotherapy, devices, and choice of stents were at the operator’s discretion. Dual antiplatelet therapy with aspirin and clopidogrel was recommended to all patients for 12 months after intervention.
Clinical, procedural, and follow-up data were prospectively collected and stored in a central database. A dedicated data-coordinating center performed all data management and analyses. Prespecified clinical and procedural data and in-hospital complications were obtained from the hospital charts, which were reviewed by independent research personnel who were unaware of the study objectives. Clinical follow-up was performed by telephone contact or office visit at 30 days, 6 months, and 1 year by trained quality assurance nurses who worked exclusively with the database to determine postintervention clinical events. Primary source documents were obtained for all events and were adjudicated by physicians not involved in the procedures and unaware of the study objectives.
The main objective of the present study was to analyze ST event rates at 1 year according to the Academic Research Consortium definitions of definite and probable. Clinical outcomes including death and myocardial infarction (MI) were also analyzed across the groups. In cases of clopidogrel discontinuation, patients were asked to provide information on the date that clopidogrel was stopped and the reasons why. Reasons for clopidogrel discontinuation were divided into (1) clinically indicated: allergy, bleeding, or surgery; (2) physician factors: physician decision or end of prescription; and (3) patient factors: lack of compliance or financial.
Definite ST was defined as angiographic or pathologic confirmation and ≥1 of the following: ischemic symptoms, ischemic electrocardiographic changes, or elevated cardiac biomarkers. Probable ST was defined as any unexplained death within 30 days or any MI with acute ischemia in the territory of stent without angiographic confirmation or other cause. Death was defined as death from any cause, cardiac and noncardiac. Cardiac death included all deaths in which a noncardiac cause could not be demonstrated. MI was defined as a total creatine kinase of ≥2 times the upper limit of normal and/or creatine kinase-MB ≥20 ng/ml, together with symptoms and/or ischemic electrocardiographic changes. Q-wave MI was defined as evidence of new pathologic Q waves in ≥2 contiguous leads on the electrocardiogram. Non-Q-wave MI was defined as MI with no new pathologic Q waves on the electrocardiogram. A major adverse cardiac event was a composite of all-cause death, MI, and definite/probable ST.
All statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina). Normally distributed continuous variables are presented as the mean ± SD. Variables not normally distributed are presented as the median ± interquartile range. Categorical variables are expressed as frequencies and percentages. Patients in first- and second-generation DES cohorts were analyzed separately. Within each DES cohort, analyses of difference among the groups according to the antiplatelet status (early ATD, late ATD, and continued) were performed using analysis of variance for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Cumulative incidences of definite ST, definite or probable ST, and major adverse cardiac events were calculated using the Kaplan-Meier method. The log-rank test was used to compare the differences in curves among the groups, with Bonferroni correction for pairwise comparisons. Values of p <0.05 were considered to be statistically significant.
Results
There were 341 patients (8.1%) in the first-generation DES cohort who discontinued clopidogrel within 1 year: early ATD <3 months in 104 patients (2.5%) and late ATD 3 to 12 months in 237 patients (5.6%); the majority (91.9%) continued clopidogrel therapy up to 1 year. In the second-generation DES cohort, 126 patients (6.2%) discontinued clopidogrel within 1 year: early ATD <3 months in 57 patients (2.8%) and late ATD 3 to 12 months in 69 patients (3.4%), whereas the majority (93.8%) continued clopidogrel therapy up to 1 year ( Figure 1 ).
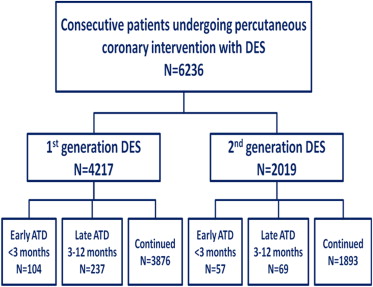
Within the first-generation DES cohort, the median days on clopidogrel for early ATD were 34 (twenty-fifth to seventy-fifth percentile 15 to 54 days) and the median days on clopidogrel for late ATD were 181 (twenty-fifth to seventy-fifth percentile 123 to 224 days). Within the second-generation DES cohort, the median days on clopidogrel for early ATD were 22 (twenty-fifth to seventy-fifth percentile 3 to 59 days) and the median days on clopidogrel for late ATD were 184 (twenty-fifth to seventy-fifth percentile 147 to 231 days).
The baseline characteristics of first- and second-generation DES cohorts are listed in Table 1 . In the first-generation DES cohort, patients who discontinued antiplatelet therapy were older and had greater prevalence of previous congestive heart failure and chronic kidney disease compared with those who continued. The second-generation DES cohort compared with the first-generation DES cohort had a greater prevalence of diabetes mellitus, previous revascularization procedures, and previous MI. Within the second-generation DES cohort, patients who discontinued antiplatelet therapy were similar except for a greater prevalence of diabetes mellitus compared with those who continued. Acute MI presentation accounted for ∼20% of both DES generation cohorts.
| Variable | First-Generation DES | Second-Generation DES | ||||||
|---|---|---|---|---|---|---|---|---|
| Early ATD <3 months (n = 104) | Late ATD 3-12 months (n = 237) | Continued (n = 3,874) | p Value | Early ATD <3 months (n = 57) | Late ATD 3-12 months (n = 69) | Continued (n = 1,893) | p Value | |
| Age, years ± SD | 70 ± 11 | 66 ± 11 | 64 ± 11 | <0.001 | 63 ± 10 | 66 ± 11 | 65 ± 11 | 0.25 |
| Men | 59 (57%) | 163 (69%) | 2,559 (66%) | 0.09 | 41 (72%) | 45 (65%) | 1,291 (68%) | 0.72 |
| Diabetes mellitus | 33 (32%) | 67 (28%) | 1,269 (33%) | 0.33 | 30 (53%) | 29 (42%) | 685 (36%) | 0.030 |
| Systemic hypertension ∗ | 90 (87%) | 186 (79%) | 3,277 (85%) | 0.029 | 52 (91%) | 56 (81%) | 1,665 (88%) | 0.17 |
| Hyperlipidemia | 86 (83%) | 206 (88%) | 3,426 (89%) | 0.12 | 56 (98%) | 57 (83%) | 1,639 (87%) | 0.022 |
| Current smoker | 18 (17%) | 51 (22%) | 780 (20%) | 0.67 | 10 (18%) | 11 (16%) | 411 (22%) | 0.40 |
| Family history of coronary artery disease | 56 (56%) | 121 (53%) | 2,025 (54%) | 0.86 | 30 (53%) | 38 (55%) | 871 (46%) | 0.22 |
| Prior percutaneous coronary intervention | 26 (26%) | 53 (23%) | 1,099 (30%) | 0.05 | 23 (40%) | 33 (49%) | 671 (36%) | 0.10 |
| Prior coronary artery bypass graft | 18 (17%) | 37 (16%) | 716 (19%) | 0.52 | 8 (14%) | 15 (22%) | 400 (21%) | 0.42 |
| Prior myocardial infarction | 20 (21%) | 39 (17%) | 761 (21%) | 0.33 | 14 (25%) | 20 (29%) | 446 (24%) | 0.61 |
| Prior congestive heart failure | 19 (19.4%) | 32 (14%) | 383 (10%) | 0.005 | 5 (9%) | 8 (12%) | 203 (11%) | 0.86 |
| Chronic kidney disease | 20 (19%) | 36 (15%) | 386 (10%) | <0.001 | 6 (11%) | 10 (15%) | 270 (14%) | 0.72 |
| Dialysis | 7 (7%) | 6 (3%) | 49 (1%) | <0.001 | 1 (2%) | 4 (6%) | 40 (2%) | 0.11 |
| Peripheral vascular disease | 12 (12%) | 43 (18%) | 536 (14%) | 0.13 | 6 (11%) | 14 (20%) | 270 (14%) | 0.27 |
| Left ventricular ejection fraction, % | 45 ± 16 | 48 ± 14 | 49 ± 14 | 0.08 | 50 ± 13 | 45 ± 19 | 47 ± 17 | 0.26 |
| Clinical presentation | ||||||||
| Stable angina pectoris | 27 (26%) | 80 (34%) | 1,194 (31%) | 0.32 | 21 (37%) | 22 (32%) | 650 (34%) | 0.84 |
| Unstable angina pectoris | 36 (35%) | 98 (42%) | 1,760 (46%) | 0.05 | 27 (47%) | 39 (57%) | 938 (50%) | 0.50 |
| Acute myocardial infarction | 23 (23%) | 44 (19%) | 774 (20%) | 0.68 | 10 (18%) | 12 (17%) | 372 (20%) | 0.83 |
| Cardiogenic shock | 4 (4.0%) | 3 (1.3%) | 76 (2%) | 0.24 | 0 | 0 | 11 (1%) | 1.00 |
| Congestive heart failure | 6 (6.1%) | 9 (4.0%) | 162 (4%) | 0.67 | 1 (2%) | 0 | 71 (4%) | 0.24 |
∗ History of systemic hypertension diagnosed and/or treated with medication or currently being treated with diet and/or medication by a physician.
The angiographic and procedural characteristics of first- and second-generation DES cohorts are listed in Table 2 . Within each DES generation cohort, there were no significant differences between patients who discontinued and those who continued antiplatelet therapy. There appears to be a greater incidence of treating type C lesions in the second-generation DES cohort. In the second-generation DES cohort, fewer stents were used per patient, stent lengths were shorter, and there was a greater incidence of bivalirudin use and fewer glycoprotein IIb/IIIa inhibitors. Intravascular ultrasound use was ∼60% in our study population.
| Variable | First-Generation DES | Second-Generation DES | ||||||
|---|---|---|---|---|---|---|---|---|
| Early ATD <3 months (patients, n = 104; lesions, n = 159) | Late ATD 3-12 months (patients, n = 237; lesions, n = 356) | Continued (patients, n = 3,874; lesions, n = 5,981) | p Value | Early ATD <3 months (patients, n = 57; lesions, n = 83) | Late ATD 3-12 months (patients, n = 69; lesions, n = 94) | Continued (patients, n = 1,893; lesions, n = 2,537) | p Value | |
| Target coronary vessel | ||||||||
| Left main | 2 (1%) | 10 (3%) | 88 (2%) | 0.13 | 1 (1%) | 0 | 56 (2%) | 0.39 |
| Left anterior descending | 63 (40%) | 139 (39%) | 2,322 (39%) | 0.98 | 30 (36%) | 27 (29%) | 947 (37%) | 0.23 |
| Left circumflex | 37 (23.3%) | 76 (21%) | 1,306 (22%) | 0.89 | 26 (31%) | 27 (29%) | 602 (24%) | 0.16 |
| Right | 53 (33.3%) | 119 (33%) | 1,920 (32%) | 0.83 | 25 (30%) | 35 (37%) | 799 (32%) | 0.48 |
| Venous graft | 3 (2%) | 10 (3%) | 320 (5%) | 0.019 | 1 (1%) | 5 (5%) | 127 (5%) | 0.30 |
| Internal mammary | 1 (0.6%) | 2 (0.6%) | 25 (0.4%) | 0.88 | 0 | 0 | 6 (0.2%) | 0.92 |
| Type C lesion | 34 (23%) | 70 (21%) | 1,216 (21%) | 0.85 | 40 (48%) | 31 (33%) | 1,173 (46%) | 0.035 |
| Restenosis lesion | 5 (3%) | 15 (4%) | 254 (4%) | 0.79 | 8 (10%) | 5 (5%) | 81 (3%) | 0.006 |
| Number of diseased vessels | 2.0 ± 0.9 | 1.8 ± 0.9 | 1.8 ± 0.9 | 0.59 | 1.7 ± 0.8 | 1.5 ± 0.7 | 1.7 ± 0.8 | 0.35 |
| Number of treated lesions | 1.5 ± 0.8 | 1.5 ± 0.8 | 1.5 ± 1.3 | 0.65 | 1.4 ± 0.6 | 1.3 ± 0.7 | 1.3 ± 0.6 | 0.46 |
| Number of stents per patient | 1.6 ± 0.9 | 1.6 ± 0.8 | 1.6 ± 0.8 | 0.89 | 1.4 ± 0.8 | 1.4 ± 0.8 | 1.5 ± 0.8 | 0.89 |
| Stent diameter, mm | 3.1 ± 0.6 | 3.2 ± 2.1 | 3.1 ± 1.3 | 0.77 | 2.9 ± 0.4 | 3.2 ± 2.7 | 3.0 ± 0.6 | 0.05 |
| Stent length, mm | 19.5 ± 6.3 | 20.3 ± 6.7 | 20.0 ± 6.4 | 0.54 | 18.3 ± 5.6 | 17.9 ± 6.1 | 18.2 ± 5.7 | 0.88 |
| Intravascular ultrasound | 103 (65%) | 223 (63%) | 3,940 (66%) | 0.53 | 54 (65%) | 46 (49%) | 1,500 (59%) | 0.08 |
| Angiographic success | 159 (100%) | 350 (99%) | 5,907 (99%) | 0.33 | 83 (100%) | 94 (100%) | 2,522 (99%) | 1.00 |
| Bivalirudin | 84 (81%) | 178 (75%) | 2,981 (77%) | 0.52 | 53 (93%) | 60 (87%) | 1,691 (89%) | 0.55 |
| Heparin | 13 (13%) | 32 (14%) | 510 (13%) | 0.97 | 4 (7%) | 9 (13%) | 199 (11%) | 0.55 |
| Glycoprotein IIb/IIIa inhibitors | 10 (10%) | 19 (8%) | 340 (9%) | 0.89 | 3 (5%) | 5 (8%) | 53 (3%) | 0.037 |
The 1-year clinical outcomes are listed in Table 3 . In the first-generation DES cohort, both early and late ATD had a greater incidence of definite and definite or probable ST compared with those who continued ( Figure 2 ). Although the incidences of ST events were numerically greater for early ATD compared with late ATD, this pairwise comparison did not demonstrate statistical difference, whereas pairwise comparisons between continued and early ATD and continued and late ATD demonstrated statistical differences. There were no ST events in early and late ATD in the second-generation DES cohort, and only 1 patient who continued therapy had definite ST ( Figure 2 ). In both the first- and second-generation DES cohorts, mortality and major adverse cardiac events were greater in early and late ATD compared with those who continued therapy ( Figure 3 ). In each generation DES cohort, pairwise comparisons between early and late ATD for both major adverse cardiac events and mortality did not demonstrate statistical differences. The cumulative event curves for definite ST ( Figure 4 ), definite or probable ST ( Figure 5 ), and major adverse cardiac events ( Figure 6 ) in first- and second-generation DES cohorts are shown.
| Variable | First-Generation DES | Second-Generation DES | ||||||
|---|---|---|---|---|---|---|---|---|
| Early ATD <3 months (n = 104) | Late ATD 3-12 months (n = 237) | Continued (n = 3,874) | p Value | Early ATD <3 months (n = 57) | Late ATD 3-12 months (n = 69) | Continued (n = 1,893) | p Value | |
| Definite stent thrombosis | 4 (4%) | 3 (1%) | 18 (0.5%) | 0.001 | 0 | 0 | 1 (0.1%) | 1.00 |
| Definite/probable stent thrombosis | 4 (4%) | 6 (3%) | 34 (0.9%) | 0.002 | 0 | 0 | 1 (0.1%) | 1.00 |
| Death | 7 (7%) | 9 (4%) | 0 | <0.001 | 3 (6%) | 5 (7%) | 0 | <0.001 |
| Cardiac death | 4 (4%) | 1 (0.4%) | 0 | <0.001 | 1 (2%) | 1 (2%) | 0 | 0.004 |
| Myocardial infarction | 2 (2%) | 5 (2%) | 30 (0.8%) | 0.035 | 1 (2%) | 1 (2%) | 29 (2%) | 0.69 |
| Q-wave myocardial infarction | 1 (1%) | 1 (0.4%) | 4 (0.1%) | 0.042 | 0 | 0 | 3 (0.2%) | 1.00 |
| Non-Q-wave myocardial infarction | 1 (1%) | 4 (2%) | 25 (0.6%) | 0.10 | 1 (2%) | 1 (2%) | 26 (1%) | 0.51 |
| Major adverse cardiac events (death, myocardial infarction, definite/probable stent thrombosis) | 10 (10%) | 13 (6%) | 36 (0.9%) | <0.001 | 3 (6%) | 5 (7%) | 4 (0.2%) | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


