The iterations of the SAPIEN prosthesis might impact the incidence and grade of paravalvular regurgitation (PVR). The aim of this study was to assess the impact of iterations of balloon-expandable valves (SAPIEN, SAPIEN XT, and SAPIEN 3) and degree of aortic valve calcification (AVC) on the severity of PVR after transcatheter aortic valve implantation (TAVI). Comprehensive echocardiographic examinations and multidetector computed tomography (MDCT) were performed in 272 patients (127 men, 81 ± 7 years old, logistic EuroScore of 21 ± 13%) who underwent TAVI with 23- and 26-mm balloon-expandable valves. The degree of AVC was assessed with MDCT. PVR grade was assessed with echocardiography. The cover index was calculated as (prosthesis area − MDCT annulus area)/prosthesis area. SAPIEN, SAPIEN XT, and SAPIEN 3 prostheses were implanted in 103 patients (38%), 105 patients (38.5%), and 64 patients (23.5%), respectively. Significant PVR (≥moderate) occurred in 14%, 10%, and 0% of patients receiving the SAPIEN, SAPIEN XT, and SAPIEN 3, respectively (p = 0.010). Across the groups, the aortic annulus size, degree of calcification, and cover index were comparable. Larger burden of AVC was independently associated with significant PVR (odds ratio 3.48, p = 0.006) after adjusting for age, body surface area, gender, aortic annulus area, cover index, and prosthesis iteration. SAPIEN 3 was associated with lower frequency of significant PVR (odds ratio 0.31, p = 0.002). In conclusion, the incidence of significant PVR significantly decreased over time with improvement in valve design. SAPIEN 3 was associated with less significant PVR after TAVI independently of the AVC burden.
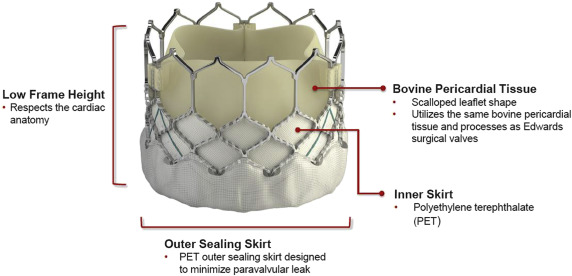
The SAPIEN 3 THV (S3-THV; Edwards Lifesciences Inc, Irvine, California) is the newest generation of balloon-expandable bioprosthetic aortic valve, which received US Food and Drug Administration approval in June 2015 for treatment of patients at high risk with severe, symptomatic aortic stenosis (AS) ( Figure 1 ). The SAPIEN 3 valve has been associated with lower incidence of significant paravalvular regurgitation (PVR) in a multicenter registry recently. The new features of this device, a new cobalt-chromium frame with large cell design containing the valve and an outer polyethylene terephthalate cuff enhance the sealing of the aortic annulus and the gaps between the prosthetic frame and the native valve caused by the presence of extensive, asymmetric bulky calcific deposits. The effect of valve iterations and degree of aortic valve calcification (AVC) on the severity of PVR after transcatheter aortic valve implantation (TAVI) has not been evaluated. The aim of this study was to investigate the impact of iteration of these balloon-expandable valves (SAPIEN, SAPIEN XT, and SAPIEN 3) and degree of AVC on the PVR severity in patients with severe AS.
Methods
From November 2007 till June 2015, all patients with symptomatic severe AS treated with balloon-expandable transcatheter aortic bioprostheses (SAPIEN, SAPIEN XT, and SAPIEN 3) at the Leiden University Medical Center were included. Patients receiving self-expandable valves, 29-mm balloon-expandable prosthesis or undergoing valve-in-valve procedures were excluded from this study. Patients deemed too high risk for surgical options with high logistic EuroSCORE and contraindications for surgery (porcelain aorta, frailty, severe chronic obstructive airway disease, and previous coronary artery bypass grafting) were referred for TAVI. Demographics, clinical, echocardiographic, multidetector row computed tomographic (MDCT) data and procedure data were prospectively collected in our center database (EPD Vision, version 8.3.3.6; Leiden, the Netherlands) for retrospective analysis.
All the TAVI candidates underwent detailed clinical and imaging workup. Either a 64-detector or 320-detector row computed tomography scanner (Aquilion 64 and Auilion ONE, respectively; Toshiba Medical Systems, Otawara, Japan) was used to assess the aortic annular size, the dimension of all the segments of the aorta, the degree and location of the AVC, and the anatomy of the peripheral arteries. The MDCT data acquisition protocol for the Aquilion 64 and Aquilion ONE has been previously described. Patients with heart rate more than 65 beats/min received either an oral beta blocker (50 to 100 mg metoprolol), unless contraindicated. Scanning was performed during midinspiratory breath-holding. The arrival of bolus media was detected using a real-time tracking technique with a threshold of +180 Hounsfield units. All the MDCT data were recorded and stored for postprocessing. Aortic annulus dimensions (perimeter, maximum diameter, minimum diameters, and aortic valve area) were measured according to the standard protocol. For quantitative assessment of AVC, Agatston score of the aortic valve was assessed (including a volume from the aortic annulus until the level of the coronary ostia including left ventricular outflow tract [LVOT]). The cover index was calculated as (prosthesis area−MDCT annulus area)/prosthesis area, as previously described. Comprehensive transthoracic echocardiography was performed before TAVI with a commercially available ultrasonographic system (Vingmed Vivid; General Electric Vingmed, Horten, Norway). Valve morphology, AS severity, and left ventricular (LV) function were measured according to the European Association of Cardiovascular Imaging/American Society of Echocardiography standards. LV ejection fraction was calculated by the Simpson’s biplane method. For quantification of aortic valve regurgitation, the jet deceleration slope (pressure halftime), jet width or jet area, vena contracta, and the diastolic flow reversal in the descending aorta were considered in an integrative approach.
TAVI was performed through transfemoral or transapical approach, based on the feasibility of the iliofemoral anatomy and suitable access sites. All procedures were performed in a fully equipped hybrid cardiac catheterization laboratory. Surgical cutdown or suture-mediated closure device (Perclose ProGlide; Abbott laboratories, Abbott Park, Illinois) were used to close the vascular access site at the femoral arteries. All procedures were performed under general anesthesia. Transesophageal echocardiography was used to support TAVI procedures, and fluoroscopy was used to guide the deployment of the valves and prosthesis positioning. Both predilatation of the native valve and prosthetic valve implantation were performed during rapid right ventricular pacing (160 to 200 beats/min) as previously described. Prosthesis position, function, and coronary ostia patency were assessed with transesophageal echocardiography and fluoroscopy. After implantation of the TAVI bioprosthesis, the presence and severity of aortic regurgitation (both transvalvular and paravalvular) was assessed with transesophageal echocardiography and classified according to the Valve Academic Research Consortium (VARC)-2 recommendations. The severity of the PVR was classified as none, trace, mild, moderate, and severe. The circumferential extend of the PVR was assessed in the parasternal short-axis views with color Doppler. The localization and severity of the PVR was judged in the midesophageal and transgastric views. Moderate or higher grade of PVR was treated with further postdilatation or second valve implantation, with agreement among the Heart Team members present during the procedure.
Continuous variables are expressed as mean ± SD or as median (interquartile range) if not normally distributed. Categorical variables are presented as frequency and percentage. For comparison of continuous variables, the Student t test, 1-way analysis of variance (with Bonferroni post hoc analysis), or the Mann–Whitney U test were used, as appropriate. For comparison of categorical variables, the chi-square test was used. Univariate and multivariate binary logistic regression analyses were performed. Odds ratios and 95% CIs were reported. Statistical analysis was performed using SPSS software, version 20 (IBM Corp, Armonk, New York). A p value <0.05 defined statistical significance.
Results
Baseline characteristics, echocardiographic features, and medication use of 272 patients (mean age 81 ± 7 years, 52% women) are presented in Table 1 . The SAPIEN valve was implanted in 103 patients (38%), 105 patients (39%) received a SAPIEN XT, and 64 patients (24%) received a SAPIEN 3. The SAPIEN 3 group had a lower percentage of patients with previous coronary artery bypass grafting, lower logistic EuroScore, more preserved LV ejection fraction, and lower number of patients using diuretics or statins than the other groups ( Table 1 ).
| Variable | Sapien (n=103) | Sapien XT (n=105) | Sapien 3 (n=64) | p-value |
|---|---|---|---|---|
| Age, (years) | 81 ± 7.5 | 81 ± 6.2 | 81 ± 5.1 | 0.756 |
| Male | 54 (52%) | 49 (47%) | 29 (45%) | 0.595 |
| Body surface area (m 2 ) | 1.82 ± 0.18 | 1.82 ± 0.18 | 1.81 ± 0.20 | 0.867 |
| Sinus rhythm/atrial fibrillation/pacemaker | 71 (69%) / 19 (18%) / 13 (13%) | 78(74%) / 16 (15%) / 11 (11%) | 42 (66%) / 16 (25%) / 6 (9%) | 0.572 |
| Hypertension | 81 (79%) | 76 (72%) | 49 (77%) | 0.566 |
| Diabetes mellitus | 31 (30%) | 26 (25%) | 19 (30%) | 0.650 |
| Hypercholesterolemia | 81 (79%) | 77 (73%) | 43 (67%) | 0.258 |
| Previous myocardial infarction | 29 (28%) | 19 (18%) | 9 (14%) | 0.061 |
| Previous coronary bypass grafting | 45 (44%) | 23 (22%) | 23 (36%) | 0.018 |
| Preoperative creatinine (μmol/L) | 108 ± 60 | 93 ± 34 | 100 ± 54 | 0.116 |
| Estimated glomerular filtration rate (ml/min/1.73m 2 ) | 63 ± 23 | 69 ± 23 | 68 ± 25 | 0.138 |
| Haemoglobin, (mmol/L) | 7.6 ± 1.0 | 7.7 ± 1.1 | 8.0 ± 1.4 | 0.057 |
| Logistic EuroSCORE (%) | 24.8 ± 13.3 | 21.0 ± 13.6 | 15.5 ± 7.9 | <0.001 |
| Echocardiography | ||||
| Left ventricular ejection fraction, % | 52.2 ± 14.3 | 55.6 ± 14.8 | 58.4 ± 15.6 | 0.029 |
| Aortic valve area, (cm 2 /m 2 ) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.994 |
| Mean aortic gradient, (mmHg) | 39.5 ± 16.0 | 43.9 ± 18.7 | 41.8 ± 15.2 | 0.164 |
| Maximal aortic gradient, (mmHg) | 64.1 ± 24.1 | 71.9 ± 28.6 | 67.4 ± 23.8 | 0.095 |
| Medications | ||||
| β-blockers | 65 (63%) | 69 (66%) | 42 (66%) | 0.911 |
| Angiotensin converting enzyme inhibitors / Angiotensin receptor blockers | 63 (61%) | 59 (56%) | 27 (42%) | 0.053 |
| Diuretics | 75 (73%) | 65 (62%) | 34 (53%) | 0.031 |
| Calcium channel blocker | 33 (32%) | 29 (28%) | 12 (19%) | 0.171 |
| Statins | 78 (76%) | 60 (57%) | 35 (55%) | 0.005 |
| Aspirin | 55 (53%) | 59 (56%) | 31 (48%) | 0.619 |
| Oral anticoagulants | 38 (37%) | 29 (28%) | 19 (30%) | 0.331 |
The MDCT and procedural characteristics for each group are illustrated in Table 2 . There were no differences in the aortic annulus area, percentage of cover index, and both mean and median Agatston calcium score of the aortic valve between the 3 groups. However, the transapical access was more frequently used in the SAPIEN and SAPIEN XT groups than in the SAPIEN 3 group. Furthermore, the 23-mm valve was more frequently used in the SAPIEN 3 group than their counterparts ( Table 2 ).
| Variable | Sapien (n=103) | Sapien XT (n=105) | Sapien 3 (n=64) | p-value |
|---|---|---|---|---|
| Transapical | 62 (60%) | 71 (68%) | 15 (23%) | <0.001 |
| Sapien Prosthesis size | 0.022 | |||
| 23 mm | 23 (22%) | 35 (33%) | 27 (42%) | |
| 26 mm | 80 (78%) | 70 (67%) | 37 (58%) | |
| Intraprocedural grade of paravalvular regurgitation ≥moderate | 14 (14%) | 10 (10%) | 0 | 0.010 |
| Aortic annulus area derived from MDCT, (mm 2 ) | 443 ± 87 | 444 ± 68 | 427 ± 65 | 0.293 |
| Cover index, (%) | 11.97 ± 15.45 | 9.52 ± 11.23 | 11.39 ± 8.48 | 0.344 |
| Agatston calcium score of the aortic valve | ||||
| Mean | 3061 ± 1468 | 3194±1981 | 3129±1449 | 0.850 |
| Median | 2771 [1940-4070] | 2723 [1806-4089] | 2860 [2129-4124] | 0.859 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


