Preprocedural chronic kidney disease and contrast-induced acute kidney injury are predictors of in-hospital death and long-term mortality. However, neither the time course of kidney function after percutaneous coronary intervention (PCI) nor the relation between the time course of kidney function and prognosis has been adequately studied. We studied 531 patients who underwent PCI for acute coronary syndrome. The continuous deterioration of kidney function (CDKF) was defined as a >25% increase in serum creatinine level or serum creatinine >0.5 mg/dl above baseline at 6 to 8 months after PCI. CDKF was observed in 87 patients (16.4%). Independent risk factors for CDKF were contrast-induced acute kidney injury, preprocedural hemoglobin level, and proteinuria. Patients with CDKF exhibited significant higher 5-year mortality rate than patients without CDKF (25% vs 9.4%, log-rank p = 0.0006). Independent risk factors for 5-year mortality were age >75 year, anemia, New York Heart Association class III or IV, low ejection fraction, and CDKF. CDKF is associated with an increased risk of all-cause mortality of 5 years in patients with acute coronary syndrome undergoing PCI.
Chronic kidney disease (CKD) is associated with cardiovascular events, such as acute coronary syndrome (ACS) and heart failure, as well as all-cause mortality. It has also been demonstrated that contrast-induced acute kidney injury (CI-AKI) that occurs after percutaneous coronary intervention (PCI) is an independent risk factor for short- and long-term mortality. These relations have been studied from the perspective of patient prognosis and have been referred to as cardiorenal syndrome. The relation between the worsening of renal dysfunction during hospitalization and the long-term outcome in patients with ACS undergoing PCI is well known. However, the time course of kidney function after discharge has not been adequately investigated, and the relation between the continuous deterioration of kidney function (CDKF) after discharge and mortality remains unknown. Therefore, we evaluated the prognosis of kidney dysfunction after discharge and long-term mortality in patients with ACS undergoing PCI.
Methods
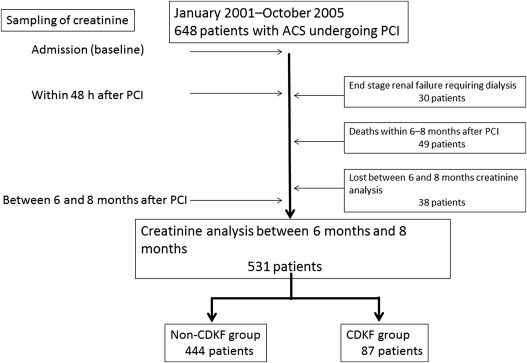
From January 2001 to October 2005, a total of 648 patients with ACS underwent PCI in our institution. Patients with end-stage renal failure requiring dialysis (n = 30), patients who died within 6 to 8 months after PCI (n = 49), and patients who failed to have serum creatinine (Cr) level measured at 6 to 8 months after PCI (n = 38) were excluded. The remaining 531 patients were divided into 2 groups: those with CDKF (CDKF group, n = 444) and those without CDKF (non-CDKF group, n = 87; Figure 1 ). The demographic and clinical characteristics of the patients were recorded on admission. Information regarding the laboratory parameters, pharmacologic data, and interventional therapies was obtained from the patient’s electronic medical charts. On admission, venous blood samples and urine samples were obtained before administration of any medications. These samples were tested using an automatic clinical chemistry analyzer (LABOSPECT 008; Hitachi, Tokyo, Japan). The Cr, total cholesterol, and high- and low-density lipoprotein levels were determined using enzymatic methods. The definition of ACS was based on the current guidelines. CDKF was defined as an absolute Cr increase of ≥0.5 mg/dl or a relative increase in Cr ≥25% above baseline from 6 to 8 months after PCI. CI-AKI was defined as an absolute Cr increase of ≥0.5 mg/dl or a relative increase in Cr ≥25% above baseline within 48 hours after PCI. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula, with coefficients modified for Japanese patients : eGFR (ml·minute −1 ·1.73 m −2 ) = 194 × serum Cr −1.094 × age −0.287 × (0.739 if female). CKD was defined as an eGFR from 15 to 59 ml·minute −1 ·1.73m −2 . Proteinuria was evaluated using a dipstick method (Ames Multistix, Bayer Diagnostics, Victoria, Australia) with a single spot urine specimen. The level of protein in the urine was recorded as (−), (+−), (1+), (2+), or (3+) and proteinuria was defined as grade (2+) or (3+). The primary end point was all-cause death after PCI. The follow-up events were conducted by trained telephone interviewers with access to the medical charts of the patients. The follow-ups were carefully monitored and recorded. PCI was performed with the femoral approach according to standard clinical practice. All patients received aspirin (162 to 200 mg) and intravenous heparin to achieve an activated clotting time of >250 seconds. None of the patients received a glycoprotein IIb/IIIa receptor antagonist. A nonionic, low-osmolality contrast medium, either iomeprol (Iomeron; Eisai, Tokyo, Japan) or iopamidol (Iopamiron; Fuji Pharmaceutical, Tokyo, Japan), was used in this study. Continuous variables are expressed as mean ± SD and categorical variables as percentage. The chi-square test and Fisher’s exact test were used to compare frequency ratios between groups. Continuous variables were compared through analysis of variance. The event-free survival curves were estimated using the Kaplan-Meier method and compared using log-rank test. Univariate and multivariate logistic regression analyses were performed to identify independent predictors of persistent renal dysfunction and all-cause mortality. A p value of <0.05 was considered statistically significant. All analyses were performed using SAS software (version 9.3; SAS Institute, Cary, North Carolina).
Results
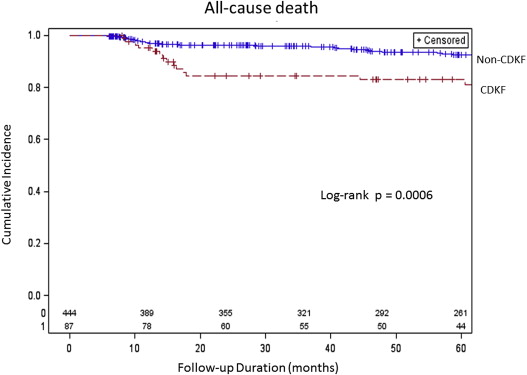
A total of 531 patients with ACS who underwent emergency PCI were divided into 2 groups: CDKF group (n = 87, 16%) and non-CDKF group (n = 444, 84%). Baseline clinical characteristics are presented in Table 1 . The CDKF group had significantly higher number of patients with diabetes mellitus, hypertension, proteinuria, pre-PCI serum Cr level of >1.5 mg/dl, and CI-AKI than the non-CDKF group. The mean preprocedural hemoglobin level and the frequency of statin use in the CDKF group were significantly lower than those in the non-CDKF group. The mean follow-up period was 60.3 ± 33 months. All-cause death within 5 years occurred in 41 patients in this study. Cardiac causes of death (n = 25) included abdominal aneurysm rupture (n = 1), fatal myocardial infarction (n = 5), death from heart failure (n = 12), arrhythmic death (n = 4), and cerebrovascular death (n = 3). Noncardiac causes of death (n = 12) included infection (n = 5) and malignancies (n = 7). The cause of death in 4 patients was unknown. The 5-year mortality in the CDKF group and the non-CDKF group was 14 patients (25%) and 27 patients (9.4%), respectively. The 5-year death from cardiac causes in the 2 groups was 13 patients (23%) and 12 patients (4.2%), respectively. All-cause death and cardiac causes of death in CDKF group were significantly higher than non-CDKF group (p = 0.001 and p <0.001, respectively). A Kaplan-Meier mortality curve is displayed in Figure 2 . The event-free survival curve was significantly higher in the CDKF group than in the non-CDKF group. The results of univariate and multivariate analyses of the predictors of CDKF are listed in Table 2 . CI-AKI, a lower level of preprocedural hemoglobin, and proteinuria were independent risk factors for CDKF. Multivariate analysis also revealed that CDKF was the strongest independent predictor of 5-year mortality ( Table 3 ).
| Variable | CDKF | p Value | |
|---|---|---|---|
| No (n = 444) | Yes (n = 87) | ||
| Men | 351 (79.1) | 64 (74) | 0.257 |
| Age (yrs) | 65.9 ± 11.9 | 68.5 ± 10.6 | 0.0594 |
| Age ≥75 yrs | 325 (73.2) | 61 (70) | 0.555 |
| Body mass index (kg/m 2 ) | 24.0 ± 3.4 | 23.5 ± 3.5 | 0.2721 |
| Hypertension | 281 (63.3) | 67 (77) | 0.0138 |
| Diabetes mellitus | 142 (32.0) | 40 (46) | 0.0119 |
| Dyslipidemia ∗ | 242 (54.5) | 47 (54) | 0.9343 |
| Total cholesterol (mg/dl) | 198 ± 38 | 201 ± 38 | 0.5856 |
| High-density lipoprotein cholesterol (mg/dl) | 49 ± 13 | 48 ± 12 | 0.6868 |
| Triglyceride (mg/dl) | 135 ± 73 | 135 ± 81 | 0.9883 |
| Low-density lipoprotein cholesterol (mg/dl) | 123 ± 33 | 124 ± 36 | 0.7029 |
| Smoker | 240 (54.1) | 46 (53) | 0.8399 |
| CKD (≥3) † | 134 (30.2) | 28 (32) | 0.7105 |
| Glomerular filtration rate (ml/min) | 69.9 ± 21.3 | 72.6 ± 28.9 | 0.4073 |
| Serum Cr value (mg/dl) | 0.90 ± 0.37 | 0.96 ± 0.70 | 0.4033 |
| Serum Cr value ≥1.5 mg/dl | 16 (3.6) | 8 (9) | 0.0217 |
| Proteinuria | 76 (17.1) | 32 (37) | <0.0001 |
| CI-AKI | 49 (11.0) | 35 (40) | <0.0001 |
| Target vessels | 1.04 ± 0.47 | 1.02 ± 0.55 | 0.7584 |
| Ejection fraction (%) | 56.0 ± 12.7 | 54.9 ± 13.0 | 0.4386 |
| Low ejection fraction (≤40%) | 58 (13.1) | 12 (14) | 0.854 |
| New York Heart Association class III or IV | 45 (10.1) | 8 (9) | 0.7892 |
| Hemoglobin (g/dl) | 13.9 ± 1.80 | 13.3 ± 1.84 | 0.0089 |
| Anemia (men <13, women <12) | 108 (24.3) | 27 (31) | 0.1887 |
| Intra-aortic balloon pump | 36 (8.1) | 6 (7) | 0.7018 |
| Contrast volume (ml) | 210 ± 63 | 212 ± 69 | 0.7764 |
| Contrast volume (>300 ml) | 41 (9.3) | 8 (9) | 0.9860 |
| Aspirin | 439 (98.9) | 87 (100) | 1.000 |
| β Blocker | 102 (23.0) | 26 (30) | 0.1681 |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 345 (77.7) | 70 (81) | 0.5693 |
| Statin | 321 (72.3) | 52 (60) | 0.0194 |
∗ Defined as low-density lipoprotein cholesterol of ≥140 mg/dl, high-density lipoprotein cholesterol of ≤40 mg/dl, and/or triglycerides of ≥150 mg/dl.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| p Value | Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | |
| Proteinuria | <0.0001 | 2.8 | 1.7–4.6 | 0.0255 | 1.9 | 1.1–3.2 |
| Hemoglobin | 0.0095 | 0.8 | 0.7–0.96 | 0.0221 | 0.9 | 0.8–0.98 |
| CI-AKI | <0.0001 | 5.4 | 3.2–9.1 | <0.0001 | 4.6 | 2.6–7.9 |
| Diabetes mellitus | 0.0069 | 1.9 | 1.2–3.1 | |||
| Serum Cr level >1.5 mg/dl | 0.0268 | 2.7 | 1.1–6.5 | |||
| Hypertension | 0.015 | 1.9 | 1.1–3.3 | |||
| Statin | 0.0204 | 0.6 | 0.4–0.9 | |||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


