The long-term risk associated with different coronary artery disease (CAD) presentations in women undergoing percutaneous coronary intervention (PCI) with drug-eluting stents (DES) is poorly characterized. We pooled patient-level data for women enrolled in 26 randomized clinical trials. Of 11,577 women included in the pooled database, 10,133 with known clinical presentation received a DES. Of them, 5,760 (57%) had stable angina pectoris (SAP), 3,594 (35%) had unstable angina pectoris (UAP) or non–ST-segment-elevation myocardial infarction (NSTEMI), and 779 (8%) had ST-segment-elevation myocardial infarction (STEMI) as clinical presentation. A stepwise increase in 3-year crude cumulative mortality was observed in the transition from SAP to STEMI (4.9% vs 6.1% vs 9.4%; p <0.01). Conversely, no differences in crude mortality rates were observed between 1 and 3 years across clinical presentations. After multivariable adjustment, STEMI was independently associated with greater risk of 3-year mortality (hazard ratio [HR] 3.45; 95% confidence interval [CI] 1.99 to 5.98; p <0.01), whereas no differences were observed between UAP or NSTEMI and SAP (HR 0.99; 95% CI 0.73 to 1.34; p = 0.94). In women with ACS, use of new-generation DES was associated with reduced risk of major adverse cardiac events (HR 0.58; 95% CI 0.34 to 0.98). The magnitude and direction of the effect with new-generation DES was uniform between women with or without ACS (p interaction = 0.66). In conclusion, in women across the clinical spectrum of CAD, STEMI was associated with a greater risk of long-term mortality. Conversely, the adjusted risk of mortality between UAP or NSTEMI and SAP was similar. New-generation DESs provide improved long-term clinical outcomes irrespective of the clinical presentation in women.
Almost 30% of patients presenting with an acute myocardial infarction (MI) are women. Women with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI) are generally older and more commonly affected by multiple co-morbidities compared with men. Across the entire spectrum of CAD, acute coronary syndromes (ACSs), characterized by acute plaque change with sudden coronary blood flow impairment, are associated with the highest rates of adverse clinical events. Previous studies demonstrated that women with ACSs have higher mortality rate and less use of therapies recommended by international guidelines compared with men. Furthermore, the long-term prognosis of women across the ACS spectrum undergoing percutaneous revascularization with DES is unclear.
Data on clinical outcomes associated with early- and new-generation DES implantation in women according to their clinical presentation are scarce as a result of their poor representation inclusion in randomized clinical trials. Therefore, we sought to evaluate, by pooling patient-level data from randomized clinical trials of DES, the prognostic impact of CAD presentation in women undergoing PCI and the safety and efficacy profile of new-generation DES in women presenting with or without ACS relative to early-generation DES.
Methods
The rationale of the present patient-level pooled database, list of trials, analytic strategies, and prespecified end points have been previously reported. Briefly, principal investigators and device manufacturers participating in the Gender Data Forum (convened on September 24, 2012, in Washington, D.C.) were contacted to obtain patient-level data for female participants from randomized trials of DES. Subsequently, we pooled patient-level data for female participants from 26 randomized clinical trials performed between 2002 and 2013 in a unique data set. The full list of randomized clinical trials included in the pooled database and their characteristics is reported in Supplementary Tables 1 and 2 .
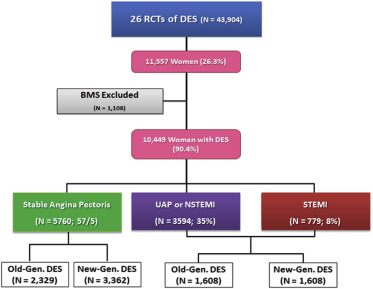
According to the clinical presentation of CAD, study population was stratified in Figure 1 : (1) stable angina pectoris (SAP), (2) unstable angina pectoris (UAP) or non–ST-segment-elevation myocardial infarction (NSTEMI), and (3) ST-segment elevation myocardial infarction (STEMI). Women randomized to bare-metal stent (BMS) implantation were excluded from the present analysis.
All trials included in our analysis complied with the provisions of the Declaration of Helsinki, and the study protocols were approved by the institutional review board at each study center. All patients provided written informed consent for participation in each study.
DES used across randomized clinical trials are described in the Supplementary Table 1 . Coronary stents were classified as early-generation DES (including sirolimus- and paclitaxel-eluting stents) and new-generation DES (including everolimus, zotarolimus stents with durable polymer and biolimus- and sirolimus-eluting stents with biodegradable polymer).
We evaluated the impact of CAD clinical presentation on 3-year all-cause mortality. Subsequently, we evaluated the effect of new-generation DES (vs early-generation DES) on the rate of 3-year major adverse cardiac events (MACE) in patients with or without ACS. MACE was defined as the composite of death, MI, or definite or probable stent thrombosis (ST). Secondary end points were the individual components of the MACE end point; the composite of death or MI; and the composite of death, MI, or definite or probable ST. The clinical end point definitions used across trials are resumed in Supplementary Table 2 .
All patient-level data were aggregated and combined as one data set on a prespecified extraction sheet. Baseline clinical, demographic, and procedural characteristics of the stent groups (i.e., BMS, early-generation DES, and newer-generation DES) were compared with linear regression for continuous variables and the chi-square tests for categorical variables. Cumulative event rates in the stent groups were calculated with the Kaplan–Meier method and compared with the log-rank test. For these analyses, the total follow-up was defined as the time from index procedure until death, last follow-up date or 3 years, whichever came first. The independent associations between clinical presentation and stent generation and outcomes were assessed with the Cox proportional hazards models that included a frailty term (γ) to assess random effects in the trials. Frailties are the unmeasured factors that affect trial-specific baseline risk and are distributed as γ random variables with a mean of 1 and variance θ. The variance parameter is interpreted as a metric of heterogeneity in baseline risk between trials. In the analysis evaluating the impact of clinical presentation on outcomes, SAP was the reference category. For the DES-level analysis, new-generation DES was the reference category. Stent group, age, and baseline variables showing significant differences between groups were included as covariates in the multivariable model (body mass index, diabetes, previous MI, family history of coronary artery disease, previous percutaneous intervention for multivessel disease, smoking, presentation with an ACS, number of stents per patient, and type B2 or C lesions). For the DES-level analysis, the consistency of the effect of new-generation DES in patients with or without ACS was evaluated with formal interaction test. We judged p values of <0.05 to be significant, and all analyses were done with SAS software.
Results
Of 11,577 women included in the pooled database, 10,133 women with a known clinical presentation were treated with a DES. Of them, 5,760 (57%) had SAP, 3594 (35%) had UAP/NSTEMI, and 779 (8%) had STEMI as clinical presentation ( Figure 1 ). Baseline clinical and angiographic characteristics are described in Table 1 . Significant heterogeneity was observed across clinical presentation in clinical and procedural variables. Women presenting with SAP had higher prevalence of diabetes mellitus, arterial hypertension, hypercholesterolemia, family history of coronary artery disease, and previous PCI. Conversely, women presenting with STEMI were more commonly smokers and less frequently had a history of previous MI and previous coronary artery bypass graft surgery. Finally, women presenting with SAP had higher prevalence of moderate- or severe-calcified lesions and bifurcation lesions.
| Variable | SAP (N = 5760) | UAP / NSTEMI (N = 3594) | STEMI (N = 779) | P-value |
|---|---|---|---|---|
| Age (years) | 67.5 ± 10.1 | 66.8 ± 11.0 | 67.0 ± 12.3 | 0.01 |
| BMI (kg / m2) | 28.6 ± 6.1 | 27.7 ± 5.6 | 26.5 ± 5.1 | < 0.01 |
| Risk Factors | ||||
| Diabetes Mellitus | 1950 (33.9%) | 1089 (30.3%) | 140 (18.0%) | < 0.01 |
| IDDM | 629 (32.3%) | 350 (32.1%) | 36 (25.7%) | 0.27 |
| Arterial Hypertension | 4550 (79.0%) | 2637 (73.4%) | 430 (55.2%) | < 0.01 |
| Hypercholesterolemia | 4180 (72.8%) | 2321 (64.6%) | 317 (40.7%) | < 0.01 |
| Serum Creatinine (mg/dl) | 0.9 ± 0.8 | 0.9 ± 0.6 | 0.9 ± 0.7 | 0.18 |
| Smoker | 1335 (23.3%) | 1080 (30.1%) | 305 (39.2%) | < 0.01 |
| Family history of CAD | 2203 (41.7%) | 1255 (35.7%) | 262 (34.2%) | < 0.01 |
| Previous Myocardial Infarction | 1048 (18.3%) | 738 (20.6%) | 69 (8.9%) | < 0.01 |
| Previous Percutaneous Coronary Intervention | 1412 (25.1%) | 641 (17.9%) | 52 (6.7%) | < 0.01 |
| Previous Coronary By-pass Graft | 310 (5.4%) | 186 (5.2%) | 10 (1.3%) | < 0.01 |
| Left Ventricular Ejection Fraction (%) | 58.1 ± 16.9 | 53.3 ± 18.6 | 47.7 ± 11.6 | < 0.01 |
| Multivessel disease | 1460 (28.7%) | 978 (30.6%) | 170 (29.9%) | 0.18 |
| Angiographic Characteristics | ||||
| Number of lesions treated | 1.3 ± 0.6 | 1.3 ± 0.7 | 1.3 ± 0.6 | < 0.01 |
| Number of stents implanted | 1.5 ± 0.9 | 1.6 ± 1.0 | 1.6 ± 0.9 | < 0.01 |
| Mean stent diameter (mm) | 2.9 ± 0.4 | 3.0 ± 0.4 | 3.1 ± 0.4 | < 0.01 |
| Total stent length (mm) | 28.9 ± 18.2 | 30.8 ± 20.4 | 32.0 ± 20.4 | < 0.01 |
| Type B2/C lesion | 2960 (61.7%) | 1841 (63.2%) | 335 (78.8%) | < 0.01 |
| Moderate/severe calcifications | 893 (26.5%) | 626 (24.9%) | 35 (14.4%) | < 0.01 |
| Bifurcation lesion | 697 (22.4%) | 207 (16.3%) | 24 (8.6%) | < 0.01 |
| Type of Stent Implanted | < 0.01 | |||
| Early-Generation DES | 2335 (40.5%) | 1330 (37.0%) | 278 (35.7%) | |
| New-Generation DES | 3425 (59.5%) | 2264 (63.0%) | 501 (64.3%) | |
A stepwise increase in 3-year rates of death ( Figure 2 ; 4.9% vs 6.1% vs 9.4%; p <0.01); death or MI (9.4% vs 10.6% vs 12.4%; p <0.01); and in the composite of death, MI, or ST ( Figure 2 ; 9.4% vs 10.8% vs 12.8%; p <0.01) was observed from SAP (lowest event rates) to STEMI (highest event rates; Table 2 ). The risk of death and of death, MI, or ST with STEMI was highest in the first year (p <0.01), whereas no differences among groups were observed in the landmark period between 1 and 3 years ( Figure 2 ). After multivariate adjustment for baseline and procedural confounders, there were no differences between SAP and UAP/NSTEMI in terms of 3-year clinical outcomes ( Table 3 ). Conversely, women with STEMI were associated with greater risk of death (hazards ratio [HR] 3.45; 95% confidence interval [CI] 1.99 to 5.98; p <0.01) compared with SAP and UAP/NSTEMI. Interestingly, women with STEMI had lower risk of MI at 3 years (HR 0.41; 95% CI 0.18 to 0.91) compared with both SAP (p = 0.03) and UAP/NSTEMI (p = 0.04). Values of the frailty parameter θ for heterogeneity were not significant for death (θ = 0.003; p = 0.19) and ST (θ = 0.01; p = 0.20); conversely, significant heterogeneity was observed for death or MI (θ = 0.22; p <0.0001) and for death, MI, or ST (θ = 0.22; p <0.0001).
| SAP (N = 5760) | UAP / NSTEMI (N = 3594) | STEMI (N = 779) | P-value ∗ | P-value † | P-value ‡ | |
|---|---|---|---|---|---|---|
| 3-Year Outcomes | ||||||
| Death | 291 (4.9%) | 219 (6.1%) | 65 (9.4%) | < 0.01 | < 0.01 | < 0.01 |
| Cardiac Death | 147 (3.5%) | 123 (3.7%) | 46 (6.4%) | < 0.01 | 0.23 | < 0.01 |
| Myocardial Infarction | 294 (5.2%) | 195 (5.6%) | 30 (4.2%) | 0.25 | 0.59 | 0.58 |
| Target Lesion Revascularization | 404 (7.4%) | 220 (6.7%) | 34 (6.9%) | 0.15 | 0.60 | 0.12 |
| Definite or Probable ST | 46 (0.7%) | 40 (1.1%) | 10 (1.8%) | 0.26 | 0.26 | 0.11 |
| Death or MI | 540 (9.4%) | 374 (10.6%) | 90 (12.4%) | < 0.01 | 0.03 | < 0.01 |
| Death or MI or ST | 545 (9.4%) | 381 (10.8%) | 92 (12.8%) | < 0.01 | 0.02 | < 0.01 |
| MACE | 845 (15.1%) | 522 (15.1%) | 110 (16.9%) | 0.23 | 0.25 | 0.09 |
| 0-1 Year Outcomes | ||||||
| Death | 102 (1.8%) | 98 (2.7%) | 49 (6.3%) | < 0.01 | < 0.01 | < 0.01 |
| Cardiac Death | 68 (1.5%) | 63 (1.8%) | 38 (4.9%) | < 0.01 | 0.48 | < 0.01 |
| Myocardial Infarction | 236 (4.1%) | 157 (4.4%) | 24 (3.1%) | 0.32 | 0.42 | 0.88 |
| Target Lesion Revascularization | 257 (4.5%) | 154 (4.3%) | 23 (3.0%) | 0.10 | 0.16 | 0.03 |
| Definite or Probable ST | 33 (0.6%) | 27 (0.8%) | 8 (1.0%) | 0.09 | 0.08 | 0.03 |
| Death or MI | 317 (5.5%) | 234 (6.5%) | 70 (9.0%) | < 0.01 | 0.03 | < 0.01 |
| Death or MI or ST | 322 (5.6%) | 240 (6.7%) | 73 (9.4%) | < 0.01 | 0.02 | < 0.01 |
| MACE | 521 (9.0%) | 348 (9.7%) | 83 (10.7%) | 0.55 | 0.64 | 0.33 |
| 1-3 Years Outcomes | ||||||
| Death | 189 (3.2%) | 121 (3.5%) | 16 (3.3%) | 0.65 | 0.38 | 0.58 |
| Cardiac Death | 79 (1.9%) | 60 (1.9%) | 8 (1.5%) | 0.82 | 0.78 | 0.58 |
| Myocardial Infarction | 58 (1.2%) | 38 (1.3%) | 6 (1.1%) | 0.77 | 0.52 | 0.47 |
| Target Lesion Revascularization | 147 (3.0%) | 66 (2.4%) | 11 (3.9%) | 0.21 | 0.07 | 0.12 |
| Definite or Probable ST | 13 (0.2%) | 13 (0.4%) | 2 (0.7%) | 0.31 | 0.14 | 0.14 |
| Death or MI | 223 (4.0%) | 140 (4.4%) | 20 (3.7%) | 0.73 | 0.43 | 0.50 |
| Death or MI or ST | 223 (4.0%) | 141 (4.4%) | 19 (3.7%) | 0.67 | 0.39 | 0.54 |
| MACE | 324 (6.6%) | 174 (5.9%) | 27 (7.0%) | 0.72 | 0.43 | 0.46 |
† p-value for SAP versus UAP/NSTEMI.
| SAP | UAP / NSTEMI HR [95% CI] | P-value ∗ | STEMI [HR, 95% CI] | P-value † | P-value ‡ | |
|---|---|---|---|---|---|---|
| Death | 1.00 | 0.99 [0.73,1.34] | 0.94 | 3.45 [1.99,5.98] | < 0.01 | < 0.01 |
| Cardiac Death | 1.00 | 0.98 [0.64,1.50] | 0.93 | 3.44 [1.69,7.01] | < 0.01 | 0.01 |
| Myocardial Infarction | 1.00 | 0.91 [0.67,1.24] | 0.56 | 0.41 [0.18,0.91] | 0.03 | 0.04 |
| Target Lesion Revascularization | 1.00 | 0.98 [0.72,1.33] | 0.88 | 0.75 [0.32,1.78] | 0.51 | 0.53 |
| Definite or Probable ST | 1.00 | 1.18 [0.64,2.15] | 0.59 | 1.12 [0.25,5.06] | 0.88 | 0.75 |
| Death or MI | 1.00 | 1.00 [0.79,1.26] | 0.98 | 1.09 [0.66,1.80] | 0.73 | 0.77 |
| Death or MI or ST | 1.00 | 1.02 [0.81,1.28] | 0.89 | 1.14 [0.69,1.87] | 0.60 | 0.63 |
| MACE | 1.00 | 0.94 [0.77,1.14] | 0.53 | 0.93 [0.60,1.46] | 0.75 | 0.62 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


