Although β blocker (BB) has constituted one of the mainstays of evidence-based therapy for patients with acute myocardial infarction (AMI), the comparative efficacy of different BBs remains uncertain. We sought to determine the comparative effectiveness of nonselective BB carvedilol and the most frequently prescribed β 1 -selective BBs (bisoprolol, metoprolol, and nebivolol) in patients with AMI undergoing percutaneous coronary intervention (PCI). A total of 7,863 patients were selected from the prospective national AMI registry, and patients were divided into carvedilol group (n = 6,231) and β 1 -selective BB group (n = 1,632) at hospital discharge. The primary end point was all-cause death or MI during follow-up. During a mean follow-up of 243 ± 144 days, all-cause death or MI occurred in 94 patients (1.5%) in the carvedilol group versus 31 patients (1.9%) in the β 1 -selective BB group (adjusted hazard ratio 0.81, 95% confidence interval 0.54 to 1.22, p = 0.32). This result was consistent across various risk subgroups. The risks of all-cause death, cardiac death, and MI were also similar between the groups. After propensity-score matching, no difference was observed in the rate of all-cause death or MI (1.7% in the carvedilol vs 1.9% in the β 1 -selective BB group, adjusted hazard ratio 0.84, 95% confidence interval 0.49 to 1.46, p = 0.55). In conclusion, no differences in the risk of all-cause death or MI were observed between the carvedilol and β 1 -selective BB groups in contemporary practice of the treatment for AMI.
The major societies of cardiology guidelines recommend that β blockers (BBs) should be used in all patients who survive an acute myocardial infarction (AMI) and do not have a contraindication. However, not all BBs are the same, as they vary in properties such as metabolic profile, receptor blockade, hemodynamics, and tolerability. The nonselective BB carvedilol is a unique vasodilating BB ; some studies suggest that carvedilol may be superior to other β 1 -selective BBs in improving left ventricular ejection fraction (LVEF) in patients with heart failure or for decreasing the incidence of postoperative atrial fibrillation after coronary artery bypass grafting. However, no large-scale studies have compared efficacy between carvedilol and β 1 -selective BBs on the basis of mortality or ischemic events in a broad spectrum of patients with AMI. Thus, we compared carvedilol against the most frequently prescribed β1-selective BBs (bisoprolol, metoprolol, or nebivolol) in AMI survivors treated with percutaneous coronary intervention (PCI) using data from an AMI-dedicated national registry.
Methods
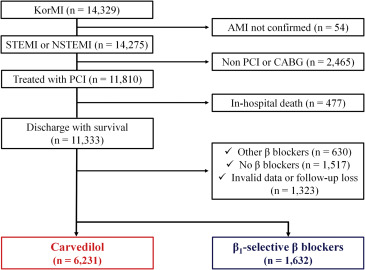
Patients with AMI registered on the Korea Working Group on Myocardial Infarction registry were analyzed for this study. From January 2008 and July 2010, a total of 14,329 consecutive patients were enrolled. The local institutional review board at each hospital approved the study protocol, and all patients were informed about their participation in the registry. ST-segment AMI is a clinical syndrome defined by characteristic symptoms of myocardial ischemia in association with persistent electrocardiographic ST elevation and subsequent release of biomarkers of myocardial necrosis. New or presumably new left bundle branch block has been considered an ST-segment AMI equivalent. In the spectrum of acute coronary syndrome, the absence of persistent ST elevation is suggestive of non–ST-segment elevation acute coronary syndrome. If cardiac biomarkers are elevated and the clinical context is appropriate, the patient is considered to have non–ST-segment elevation AMI. Inclusion criteria for the present analysis were (1) consecutive patients aged ≥18 years; (2) patients with ST-segment elevation or non–ST-segment elevation AMI at the index hospitalization; and (3) patients undergoing PCI. Exclusion criteria were (1) inhospital coronary artery bypass grafting; (2) inhospital death; (3) prescribed other BBs or none; and (4) invalid data or follow-up loss. From registered patients, we ultimately included 7,863 in this analysis; patients were divided into carvedilol group and β1-selective BB group according to the use of BBs at discharge ( Figure 1 ).
Coronary interventions were performed according to current guidelines. After the procedure, the patients were maintained on 100- to 200-mg aspirin indefinitely. Clopidogrel (75 mg/day) was prescribed as per existing guidelines. The follow-ups in the outpatient clinic occurred immediately after hospital discharge, 1 month after discharge, and at intervals of <6 months thereafter. All events were identified by the patient’s physician and confirmed by the principal investigator of each hospital.
Clinical events were defined according to a report of the American College of Cardiology Foundation/American Heart Association task force on clinical data standards. All deaths were considered cardiac in origin unless a noncardiac origin was definitely documented. MI was defined as recurrent symptoms with new electrocardiographic changes compatible with AMI or cardiac-specific marker levels at least greater than the ninety-ninth percentile upper reference limit as the reference standard. The primary end point was a composite of all-cause death or MI at the clinical follow-up. The secondary outcomes were all-cause death, cardiac death, and MI. Estimated creatinine clearance was calculated according to the Cockcroft–Gault formula.
Baseline characteristics of patients were described using median with interquartile range (IQR) or mean ± standard deviation for continuous variables and frequencies and percentages for categorical variables. Continuous variables were compared with the Student t test or the Mann–Whitney U test, and categorical variables were compared by the chi-square or Fisher’s exact statistics, as appropriate. Cumulative event rates were estimated by the Kaplan–Meier method and assessed by the log-rank statistic. Differences in the risks of the primary end point and individual clinical outcomes were investigated by multivariate Cox proportional hazards models between the carvedilol and β 1 -selective BBs groups using backward stepwise selection and expressed as adjusted hazard ratios (HR) and their 95% confidence intervals (CI), respectively.
Because the groups were not randomized, the present study was also designed using propensity-score matching to assemble a balanced cohort in which patients receiving carvedilol or β 1 -selective BBs would be balanced for baseline characteristics. The probability of receiving carvedilol rather than β 1 -selective BBs was estimated using a nonparsimonious multivariate logistic regression model in which the receipt of carvedilol was the independent variable, and all baseline characteristics as listed in Tables 1 and 2 were used as covariates. The discrimination abilities of the propensity-score model were assessed with C -statistic. A 1:1 individual matching (1,614 in the carvedilol group vs 1,614 in the β 1 -selective BB group) without replacement using the propensity score was used. The covariate balance achieved by matching was assessed by calculating the absolute standardized mean differences in covariates between the use of carvedilol and β 1 -selective BBs. An absolute standardized mean difference of <10.0% for the measured covariate suggests appropriate balance between the 2 groups. In the propensity score–matched population, continuous variables were compared with a paired t test or the Wilcoxon signed-rank test, and categorical variables were compared with the McNemar or Bhapkar test, as appropriate. Multivariate stratified Cox regression models adjusted for the available variables including all variables with univariate p <0.05 and carvedilol versus β 1 -selective BBs at discharge were used to assess adjusted hazard ratios with 95% confidence intervals of the impact of carvedilol treatment on the primary end point and individual clinical outcomes in the propensity-score matched population. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, North Carolina) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria, URL http://www.R-project.org/ ). A 2-sided p value <0.05 was considered significant.
| Overall population (n = 7,863) | Matched population (n =3,228 ) | |||||
|---|---|---|---|---|---|---|
| Carvedilol (n = 6,231) | β1-selective BBs (n = 1,632) | p Value | Carvedilol (n=1,614) | β1-selective BBs (n=1,614) | p Value | |
| Age (years) | 64.8±12.4 | 64.4±12.5 | 0.22 | 64.4±12.5 | 64.0±12.3 | 0.38 |
| Male | 4604 (73.9%) | 1176 (72.1%) | 0.20 | 1174 (72.7%) | 1168 (72.4%) | 0.81 |
| BMI (kg/m2) | 24.0±2.9 | 24.3±3.0 | 0.43 | 24.2±3.3 | 24.2±3.0 | 0.48 |
| Medical History | ||||||
| Hypertension | 3046 (48.9%) | 831 (50.9%) | 0.15 | 825 (51.1%) | 824 (51.1%) | 0.97 |
| Diabetes | 1638 (26.3%) | 444 (26.7%) | 0.35 | 454 (28.1%) | 441 (27.3%) | 0.60 |
| Systolic BP (mmHg) | 129.9±27.9 | 130.0±28.3 | 0.81 | 131.3±30.1 | 130.0±28.2 | 0.20 |
| Heart rate (beat/min) | 76.8±18.1 | 77.6±19.4 | 0.12 | 78.0±19.2 | 77.6±19.4 | 0.40 |
| STE AMI | 3850 (61.8%) | 943 (57.8%) | 0.003 | 689 (42.7%) | 680 (42.1%) | 0.74 |
| Killip class ≥II | 1473 (23.6%) | 444 (27.2%) | 0.003 | 402 (24.9%) | 439 (24.2%) | 0.14 |
| Atrial fibrillation | 169 (2.7%) | 40 (2.5%) | 0.55 | 37 (1.1%) | 40 (1.2%) | 0.73 |
| LVEF (%) | 58.4±20.7 | 52.7±13.4 | 0.16 | 52.7±13.4 | 52.2±17.4 | 0.30 |
| RWMS | 19.2±8.8 | 21.2±8.1 | <0.001 | 21.08±8.71 | 21.19±8.14 | 0.70 |
| Moderate to severe MR | 123 (2.0%) | 18 (1.1%) | 0.018 | 25 (1.5%) | 18 (1.1%) | 0.28 |
| Glucose at admission (mg/dL) | 146 (120-191) | 146 (119-195) | 0.54 | 167.06±74.76 | 166.43±74.19 | 0.80 |
| eCrCl (dL/min) | 74.5±30.1 | 75.5±30.2 | 0.24 | 75.5±30.0 | 75.2±29.6 | 0.79 |
| HDL-C (mg/dL) | 43.6±13.0 | 44.3±16.7 | 0.12 | 44.23±16.38 | 44.26±16.70 | 0.95 |
| LDL-C (mg/dL) | 118.3±37.1 | 119.8±37.2 | 0.13 | 119.7±37.1 | 119.0±38.3 | 0.59 |
| hs-CRP (mg/dL) | 0.52 (0.20-1.87) | 0.52 (0.19-1.95) | 0.001 | 4.33±15.2 | 4.54±16.4 | 0.71 |
| CK-MB (ng/ml) | 59.2 (13.2-179.5) | 54.1 (9.0-160.2) | 0.02 | 117.0±216.9 | 118.3±169.5 | 0.84 |
| Overall population (n = 7,863) | Matched population (n =3,228 ) | |||||
|---|---|---|---|---|---|---|
| Carvedilol (n = 6,231) | β1-selective BBs (n = 1,632) | p Value | Carvedilol (n=1,614) | β1-selective BBs (n=1,614) | p Value | |
| Culprit coronary artery | ||||||
| Left main | 126 (2.0%) | 15 (0.9%) | 0.001 | 21 (1.3%) | 15 (0.9%) | 0.32 |
| LAD | 3058 (49.1%) | 761 (46.6%) | 0.08 | 757 (46.9%) | 753 (46.7%) | 0.89 |
| LCx | 1021 (16.4%) | 283 (17.3%) | 0.37 | 269 (16.7%) | 281 (17.4%) | 0.57 |
| RCA | 1978 (31.7%) | 559 (34.3%) | 0.06 | 555 (34.4%) | 553 (34.3%) | 0.94 |
| Anterior AMI | 3184 (51.1%) | 776 (47.5%) | 0.011 | 778 (48.2%) | 768 (47.6%) | 0.55 |
| Multi-vessel coronary disease | 3337 (53.6%) | 984 (58.1%) | 0.001 | 933 (57.8%) | 938 (58.1%) | 0.86 |
| Pre TIMI score | <0.001 | 0.11 | ||||
| 0 | 3363 (54.0%) | 944 (57.8%) | 919 (28.5%) | 931 (28.8%) | ||
| 1 | 617 (9.9%) | 200 (12.3%) | 165 (5.1%) | 200 (6.2%) | ||
| 2 | 811 (13.0%) | 179 (11.0%) | 187 (5.8%) | 178 (5.5%) | ||
| 3 | 1440 (23.1%) | 309 (18.9%) | 343 (10.6%) | 305 (9.4%) | ||
| Procedural modality | ||||||
| Stent | 5807 (93.2%) | 1507 (92.3%). | 0.22 | 1491 (92.4%) | 1490 (92.3%) | 0.94 |
| BMS | 504 (8.1%) | 119 (7.3%) | 0.28 | 124 (3.8%) | 119 (3.7%) | 0.73 |
| 2 nd generation DESs | 3171 (54.6%) | 735 (45.0%) | <0.001 | 741 (45.9%) | 715 (44.3%) | 0.36 |
| Diameter of stent (mm) | 3.14±0.44 | 3.17±0.47 | 0.027 | 3.18±0.45 | 3.17±0.47 | 0.71 |
| Length of stent (mm) | 23.72±6.70 | 24.30±7.05 | 0.003 | 24.18±6.74 | 24.26±7.04 | 0.72 |
| Discharge medications | ||||||
| Aspirin | 6178 (99.1%) | 1615 (99.0%) | 0.46 | 1596 (98.9%) | 1597 (98.9%) | 0.87 |
| Thienopyridine | 6150 (98.7%) | 1607 (98.5%) | 0.47 | 1585 (98.2%) | 1591 (98.6%) | 0.40 |
| ACEI or ARB | 5404 (86.7%) | 1429 (87.6%) | 0.37 | 1431 (88.7%) | 1414 (87.6%) | 0.36 |
| Statin | 5240 (84.1) | 1310 (80.3) | <0.001 | 1311 (81.2) | 1298 (80.4) | 0.56 |
| Spironolactone | 486 (7.8) | 97 (5.9) | 0.011 | 99 (6.1) | 97 (6.0) | 0.88 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


