Transcatheter aortic valve replacement (TAVR) has emerged as an alternative treatment for surgical high-risk patients with severe aortic stenosis. The aim of this study was to determine the impact of atrial fibrillation (AF) on procedural outcomes. Data from 137 patients who underwent TAVR using Edwards SAPIEN valve were reviewed. The predictors of new-onset atrial fibrillation (NOAF) after the procedure were analyzed. In addition, the post-TAVR clinical outcomes and adverse events were compared according to the presence and absence of preprocedural and postprocedural AF. Previous AF was present in 49% of the patients who underwent TAVR. After the procedure, NOAF was detected in 21% of patients, and the cumulative incidence of post-TAVR AF was 60%. After TAVR, 50% of all the episodes of NOAF occurred in the initial 24 hours after the procedure. Transapical approach was observed to an important predictor of NOAF (adjusted odds ratio [OR] 5.05, 95% confidence interval [CI] 1.40 to 18.20, p = 0.013). The composite outcome of all-cause mortality, stroke, vascular complications, and repeat hospitalization in 1 month after TAVR was significantly higher in patients with previous AF (33 of 67 vs 19 of 70, adjusted OR 2.60, 95% CI 1.22 to 5.54, p = 0.013) compared with patients who did not have previous AF. The presence of post-TAVR AF led to a prolongation in the duration of intensive care unit stay by an average of 70 hours (95% CI 25 to 114.7 hours, p = 0.002). Similarly, post-TAVR AF also led to the prolongation in the hospital stay by an average of 6.7 days (95% CI 4.69 to 8.73 days, p <0.0005). In conclusion, our study demonstrates that the presence of AF before TAVR is an important predictor of the composite end point of all-cause mortality, stroke, vascular complications, and repeat hospitalization in 1 month after the procedure. AF after TAVR is more likely to be encountered with the transapical approach and is associated with a prolongation of intensive care unit and hospital stay.
Transcatheter aortic valve replacement (TAVR) is a relatively new procedure that has evolved as an alternative treatment option for patients with severe aortic stenosis (AS) who are at a high surgical risk. Atrial fibrillation (AF) is the most common cardiac arrhythmia with an increased prevalence especially in the elderly population. Because of the relatively older age of patients with severe AS, the prevalence of AF in this patient population tends to be higher. The presence of severe AS also leads to left ventricular hypertrophy and a state of an increased afterload; both these pathophysiological processes also mediate the development of AF. Both the presence and development of AF have been observed to be associated with a higher incidence of adverse clinical outcomes and increased mortality in the setting of surgical aortic valve replacement and cardiothoracic surgeries. AF carries an increased risk of thromboembolic and vascular complications compared with patients in sinus rhythm (SR). AF also leads to an atrioventricular dyssynchrony, which further adversely affects cardiac function in AS. The aim of this study was to determine the impact of AF on procedural outcomes.
Methods
The study population consisted of 137 consecutive patients with severe AS who under underwent TAVR at Massachusetts General Hospital between June 2008 and October 2012. The procedure was performed using the balloon-expandable valve (Edwards SAPIEN, SAPIEN XT; Edward Lifesciences, Irvine, California). The preprocedural risk was calculated using the Society of Thoracic Surgeons risk score for Prediction of Mortality and the logistic EuroSCORE. The study design is briefly explained in Figure 1 . Data on baseline characteristics on all patients were collected retrospectively. Preprocedural and postprocedural electrocardiograms (ECGs) were analyzed for rhythm, various intervals, and conduction abnormalities. Data on various echocardiographic parameters were obtained from the transthoracic echocardiograms that were obtained before and after TAVR.
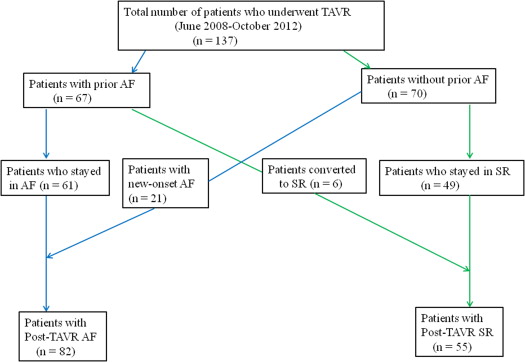
Patients were evaluated for the presence of AF before the procedure. The presence of AF was confirmed on ECGs and the documentation from inpatient and outpatient medical records. Pre-TAVR rhythm was documented on all patients who underwent the procedure. All the study patients were monitored by continuous telemetry until the day of discharge. New-onset atrial fibrillation (NOAF) was defined as any episode of AF lasting >30 seconds in the patients without any history of this arrhythmia. In the patients who developed NOAF, collected data included the timing of arrhythmia occurrence, requirement of cardioversion, and the use antithrombotic therapy. The patients were divided into 2 groups: post-TAVR AF (all patients with AF ≥30 seconds post-TAVR) and post-TAVR SR.
Univariate analyses were performed for comparison between various subgroups (previous AF vs no previous AF, post-TAVR AF vs post-TAVR SR). Categorical variables between the 2 subgroups were compared using the Fisher’s exact test, and continuous variables were compared using the 2-tailed unpaired Student’s t test. After the univariate analyses, 4 logistic regression models were prepared for each of the adverse outcomes (NOAF, major vascular complication, composite outcome of all-cause mortality, stroke, vascular complications, and repeat hospitalization in 1 month after TAVR, and all-cause 1-year mortality). We also prepared 2 linear regression models (each for duration of intensive care unit [ICU] stay and duration of hospital stay, respectively) to investigate the impact of post-TAVR AF on these outcomes. The logistic regression models were all tested for the “goodness of fit” of the logistic model with the Hosmer–Lemeshow test. All statistical tests were performed with STATA 9.2 software (Stata Corp LP, College Station, TX).
Results
The median age was 85 years (range 55 to 96). Previous AF was present in 67 patients (49%). There was no difference in the baseline clinical variables in the patients with or without previous AF, except that the use of warfarin was significantly more common in the patients with previous AF (59.7% vs 1.4%; p <0.0001). The baseline characteristics of study patients and the comparison between the patients according to the presence of previous AF are listed in Table 1 . ECG performed at the time of TAVR revealed the presence of AF in 60% (40 of 67) of the patients with previous AF. The comparison of various echocardiographic parameters between the 2 groups of patients (previous AF vs without previous AF) is listed in Table 1 . TAVR was performed using the Edwards SAPIEN (Edwards Lifesciences) balloon-expandable prosthesis with the 2 available sizes (23 and 26 mm). Transapical approach was used in most patients (81 of 137; 59%).
| Baseline variables | Study population (N = 137) | No Prior AF (N = 70) | Prior AF (N = 67) | P value |
|---|---|---|---|---|
| Age (years) | 84.18 ± 6.83 | 83.92 ± 6.96 | 84.46 ± 6.72 | 0.65 |
| Male | 65 (47%) | 30 (43%) | 35 (52%) | 0.31 |
| Body mass index (kg/m 2 ) | 26.70 ± 5.90 | 26.40 ± 5.87 | 27 ± 5.94 | 0.55 |
| Active smokers | 7 (5%) | 5 (7%) | 2 (3%) | 0.44 |
| STS-PROM score (%) | 6.88 ± 3.82 | 6.49 ± 3.18 | 7.29 ± 4.38 | 0.23 |
| Logistic EuroSCORE (%) | 14.33 ± 12.24 | 13.67 ± 12.01 | 15.02 ± 12.52 | 0.52 |
| Hypertension | 109 (80%) | 54 (77%) | 55 (82%) | 0.53 |
| Hyperlipidemia | 95 (69%) | 49 (70%) | 46 (69%) | 1.00 |
| Diabetes Mellitus | 47 (34%) | 27 (39%) | 20 (40%) | 0.37 |
| Coronary artery disease | 99 (72%) | 48 (69%) | 51 (76%) | 0.35 |
| Congestive heart failure | 74 (54%) | 34 (39%) | 40 (60%) | 0.23 |
| Chronic obstructive pulmonary disease | 39 (28%) | 18 (26%) | 21 (31%) | 0.57 |
| Peripheral vascular disease | 42 (31%) | 24 (34%) | 18 (27%) | 0.36 |
| Pulmonary hypertension | 38 (28%) | 14 (20%) | 24 (36%) | 0.06 |
| Serum creatinine (mg/dl) | 1.33 ± 0.47 | 1.32 ± 0.49 | 1.34 ± 0.45 | 0.80 |
| Cerebrovascular disease | 25 (18%) | 15 (21%) | 10 (15%) | 0.38 |
| Carotid artery disease | 31 (23%) | 20 (29%) | 11 (16%) | 0.10 |
| Previous Procedural | ||||
| Percutaneous coronary intervention | 51 (37%) | 23 (33%) | 28 (42%) | 0.29 |
| Prior pacemaker implantation | 27 (20%) | 12 (17%) | 15 (22%) | 0.52 |
| Balloon valvuloplasty | 24 (18%) | 13 (19%) | 11 (16%) | 0.83 |
| Coronary artery bypass grafting | 55 (40%) | 24 (34%) | 31 (46%) | 0.17 |
| Prior Medication h/o | ||||
| Aspirin | 113 (82%) | 64 (91%) | 49 (73%) | 0.007 |
| Clopidogrel | 28 (20%) | 16 (23%) | 12 (18%) | 0.53 |
| Warfarin | 41 (30%) | 1 (1%) | 40 (60%) | <0.0001 |
| Beta-blockers | 90 (66%) | 44 (63%) | 46 (69%) | 0.74 |
| Diuretics | 108 (79%) | 56 (80%) | 52 (78%) | 0.84 |
| ACEi/ARBs | 55 (40%) | 27 (39%) | 28 (42%) | 0.73 |
| Statins | 106 (79%) | 55 (79%) | 51 (76%) | 0.84 |
| Echocardiographic parameters | ||||
| Ejection fraction (%) | 55.29 ± 17.10 | 55.10 ± 17.95 | 56.25 ± 13.87 | 0.89 |
| LVEF < 40% | 32 (23%) | 19 (27%) | 13 (19%) | 0.32 |
| LV EDD (mm) | 44.58 ± 7.14 | 43.81 ± 7.16 | 45.54 ± 7.11 | 0.16 |
| Severe mitral regurgitation | 8 (6%) | 4/70 (6%) | 4/67 (6%) | 1.00 |
| Moderate mitral regurgitation | 60 (44%) | 24/66 (36%) | 36/63 (57%) | 0.022 |
| Mean aortic gradient (mm Hg) | 50.73 ± 16.29 | 54.36 ± 16.56 | 46.94 ± 15.22 | 0.0072 |
| Peak aortic gradient (mm Hg) | 89.60 ± 26.53 | 92.20 ± 25.51 | 80.75 ± 26.49 | 0.011 |
| Left atrial size (mm) | 43.76 ± 6.71 | 41.40 ± 6.37 | 46.24 ± 6.17 | <0.0001 |
| Interventricular septum (mm) | 13.38 ± 2.09 | 13.40 ± 2.14 | 13.36 ± 2.05 | 0.91 |
| Aortic valve area (cm 2 ) | 0.60 ± 0.13 | 0.58 ± 0.13 | 0.60 ± 0.13 | 0.27 |
To determine the incidence of NOAF, the 67 patients with a history of AF were excluded. In the remaining 70 patients, NOAF occurred in 21 patients (21 of 70; 30%). A total of 50% of all the episodes of NOAF occurred in the initial 24 hours after TAVR ( Figure 2 ). Electrical cardioversion was performed in 3 patients who developed NOAF, and 8 patients converted from AF back to sinus rhythm after receiving amiodarone. The comparison of baseline clinical, echocardiographic, and procedural characteristics of the patients categorized according to the occurrence of NOAF is listed in Table 2 . In the multivariate analysis, transapical approach of TAVR was observed to be an important predictor of NOAF after the procedure (adjusted odds ratio [OR] 5.05, 95% confidence interval [CI] 1.40 to 18.20; p = 0.013).
| Variables | New onset AF (n = 21) | SR (n = 49) | Odds ratio and 95% CI (Univariate analysis) | P value |
|---|---|---|---|---|
| Age (years) | 84.48 ± 6.01 | 83.69 ± 7.38 | 0.67 | |
| Female | 15 (71%) | 27 (55%) | 2.04; 0.68-6.13 | 0.29 |
| Body mass index (Kg/m 2 ) | 25.62 ± 5.8 | 26.73 ± 5.9 | 0.47 | |
| Active smokers | 1 (5%) | 4 (8%) | 0.56; 0.06-5.36 | 1.00 |
| Hypertension | 16 (76%) | 38 (78%) | 0.93; 0.28-3.10 | 1.00 |
| Hyperlipidemia | 13 (62%) | 36 (73%) | 0.59; 0.20-1.74 | 0.35 |
| Diabetes mellitus | 6 (29%) | 21 (43%) | 0.53; 0.18-1.61 | 0.29 |
| Coronary artery disease | 13 (62%) | 35 (71%) | 0.65; 0.22-1.91 | 0.57 |
| H/o Percutaneous coronary intervention | 6 (29%) | 17 (35%) | 0.75; 0.25-2.30 | 0.78 |
| H/o Coronary artery bypass grafting | 7 (33%) | 17 (35%) | 0.94; 0.32-2.78 | 1.00 |
| Cerebrovascular disease | 6 (29%) | 9 (18%) | 1.78; 0.54-5.85 | 0.35 |
| Congestive heart failure | 10 (48%) | 24 (49%) | 0.95; 0.34-2.64 | 1.00 |
| Peripheral Vascular disease | 6 (29%) | 18 (37%) | 0.69; 0.22-2.10 | 0.59 |
| H/o Balloon aortic valvuloplasty | 4 (19%) | 9 (18%) | 1.05; 0.28-3.87 | 1.00 |
| Chronic obstructive Pulmonary disease | 5 (24%) | 13 (27%) | 0.87; 0.26 -2.84 | 1.00 |
| Serum creatinine (mg/dl) | 1.34 ± 0.48 | 1.32 ± 0.49 | 0.86 | |
| EGFR (<60 ml/min) | 15 (71%) | 27 (55%) | 2.04; 0.68-6.13 | 0.29 |
| Prior Medication h/o | ||||
| Aspirin | 19 (90%) | 45 (92%) | 0.84; 0.14-5.01 | 1.00 |
| Clopidogrel | 6 (29%) | 10 (20%) | 1.56; 0.48-5.05 | 0.54 |
| Beta-Blockers | 12 (57%) | 32 (65%) | 0.71; 0.25-2.02 | 0.59 |
| ACEi/ARBs | 7 (33%) | 20 (41%) | 0.73; 0.25-2.12 | 0.60 |
| Statins | 17 (81%) | 38 (78%) | 1.23; 0.34-4.42 | 1.00 |
| Echocardiographic parameters | ||||
| Ejection fraction (%) | 55.95 ± 18.71 | 54.71 ± 17.80 | 0.79 | |
| Ejection fraction (<40%) | 6 (29%) | 13 (26%) | 1.11; 0.35-3.46 | 1.00 |
| Left ventricular end diastolic diameter (mm) | 43.43 ± 8.27 | 43.98 ± 6.72 | 0.77 | |
| Severe Mitral regurgitation | 3 (14%) | 1 (2%) | 8.0; 0.78-82.03 | 0.077 |
| Mean Aortic Gradient (mm Hg) | 52.28 ± 13.51 | 55.24 ± 17.76 | 0.49 | |
| Aortic valve area (cm 2 ) | 0.58 ± 0.14 | 0.57 ± 0.12 | 0.87 | |
| Left atrial size (mm) | 40.47 ± 7.39 | 41.77 ± 5.94 | 0.43 | |
| Right ventricular systolic Pressure (mm Hg) | 50.57 ± 15.16 | 47.35 ± 11.27 | 0.33 | |
| Transapical approach | 18 (86%) | 21 (43%) | 8.0; 2.1-30.77 | 0.0013 |
The overall 1-year mortality in this study was 12% (16 patients). The standard definition of “improvement in functional status and health-related quality of life after the procedure” was used for determining the success of TAVR. According to this definition, the procedural success of TAVR was 95%. Based on the predischarge echocardiogram, there was a significant decrease in the mean (50.73 ± 16.29 to 11.67 ± 4.52 mm Hg; p <0.001) and peak aortic gradients (89.60 ± 26.53 to 23.18 ± 8.57 mm Hg; p <0.001) after the procedure. There was no significant difference in 1-year post-TAVR mortality in the patients according to the presence of previous AF (15% in patients with previous AF vs 9% in patients without previous AF; OR 1.87, 95% CI 0.64 to 5.48, p = 0.29) on univariate analysis. The univariate comparison of clinical outcomes, adverse events, and echocardiographic outcomes in patients grouped according to the presence of previous AF is described in Table 3 . The incidence of the combined end point of all-cause 1-year mortality, stroke, vascular complications, and repeat hospitalization in 1-month post-TAVR was also analyzed. On multivariate analysis using logistic regression, previous AF was detected to be an important predictor of this combined end point (adjusted OR 2.60, 95% CI 1.22 to 5.54, p = 0.013).
| Clinical Outcomes | No h/o AF (n = 70) | Pre-existing AF (n = 67) | OR with 95 % CI (Univariate Analysis) | p value |
|---|---|---|---|---|
| AF at the time of procedure | 0/70 | 40 (60%) | 207.65; 12.33-3498.3 | <0.0001 |
| Procedural success | 67 (96%) | 63 (94%) | 0.71; 0.15-3.28 | 0.71 |
| Valve related complication (embolization, damage to the valve and requirement of 2nd valve) | 4 (6%) | 2 (3%) | 0.51; 0.089-2.869 | 0.68 |
| Post procedural AF | 21 (30%) | 61 (91%) | 23.72; 8.88-63.36 | <0.001 |
| Myocardial Infarction | 1 (1%) | 1 (1%) | 1.05; 0.06-17.07 | 1.00 |
| Development of new conduction block | 22 (31%) | 22 (33%) | 1.07; 0.52-2.19 | 1.00 |
| Implantation of new pacemaker | 20 (27%) | 11 (16%) | 0.49; 0.21-1.13 | 0.10 |
| Major vascular complication | 2 (3%) | 6 (9%) | 3.34; 0.65-17.20 | 0.16 |
| Cerebrovascular events (all) | 4 (6%) | 3 (5%) | 0.77; 0.17- 3.60 | 1.00 |
| Stroke | 2 (3%) | 3 (5%) | 1.60; 0.26- 9.90 | 0.68 |
| Repeat hospitalization in 1 month | 9/67 (13%) | 14/61 (23%) | 0.52; 0.21-1.31 | 0.18 |
| Death within 1 month | 3 (4%) | 6 (9%) | 2.20; 0.53-9.17 | 0.32 |
| Death after 1 month | 3/67 (5%) | 4/61 (7%) | 1.50; 0.32-6.98 | 0.71 |
| Overall Mortality | 6/70 (9%) | 10/67 (15%) | 1.87; 0.64-5.48 | 0.29 |
| Overall Mortality secondary to cardiac causes | 1/70 (1%) | 7/67 (10%) | 8.05; 0.96- 67.35 | 0.031 |
| Length of hospitalization (days) | 11.12 ± 6.36 | 15.67 ± 8.12 | 0.0005 | |
| Composite of clinical outcomes (All-cause mortality, stroke, vascular complications and repeat hospitalization in 1 month) | 19(27%) | 33 (49%) | 2.61; 1.28-5.31 | 0.009 |
| (All-cause mortality, stroke and vascular complications) | 11 (16%) | 20 (30%) | 2.28; 0.995-5.233 | 0.066 |
| LVEF prior to discharge from the hospital (%) | 57.51 ± 15.07 | 56.25 ± 13.87 | 0.62 | |
| Mean Ao gradient (mm Hg) | 12.45 ± 4.56 (n = 67) | 10.86 ± 4.41 (n = 63) | 0.0452 | |
| Peak Ao gradient (mm Hg) | 24.99 ± 8.83 (n = 67) | 21.27 ± 7.90 (n = 63) | 0.0126 | |
| Δ Mean Aortic gradient (mm Hg) | 41.91 ± 16.32 (n = 67) | 36.27 ± 14.19 (n = 63) | 0.0372 | |
| Δ Peak Aortic gradient (mm Hg) | 66.93 ± 23.89 (n = 67) | 59.97 ± 24.92 (n = 63) | 0.1071 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


