The aim of this study was to assess the impact of baseline anemia on the outcomes of patients with ST elevation myocardial infarctions who underwent primary percutaneous coronary intervention in relation to contemporary adjunctive antithrombotic therapy and gender. In the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, patients were randomized to bivalirudin alone or to unfractionated heparin plus a glycoprotein IIb/IIIa inhibitor before primary percutaneous coronary intervention. Outcomes were assessed at 30 days and 1 year according to anemia and gender. Baseline anemia was present in 331 of 3,153 patients (10.5%). Patients with versus without baseline anemia had a more than twofold increase in major bleeding at 30 days (13.5% vs 6.7%, p <0.0001) and at 1 year (14.8% vs 7.2%, p <0.0001), an association that on multivariate analysis was independent of gender. Mortality was significantly higher in men with versus without baseline anemia (4.6% vs 1.8% at 30 days, p = 0.003; 8.9% vs 3.0% at 1 year, p <0.0001) but not in women (5.3% vs 3.6% at 30 days, p = 0.42; 7.5% vs 5.9% at 1 year, p = 0.54). On multivariate analysis, anemia independently predicted 1-year all-cause mortality in men but not in women. Bivalirudin compared with unfractionated heparin plus a glycoprotein IIb/IIIa inhibitor resulted in twofold lower rates of all-cause and cardiac mortality and major bleeding in patients without but not in those with baseline anemia. In conclusion, baseline anemia was associated with increased major bleeding and death in patients with ST elevation myocardial infarctions who underwent primary PCI but was a stronger predictor of early and late mortality in men than in women. Paradoxically, in this post hoc analysis, the reductions in major bleeding and mortality in ST elevation myocardial infarction afforded by bivalirudin occurred primarily in patients without baseline anemia.
Anemia is common in patients admitted with acute coronary syndromes, including ST elevation myocardial infarctions (STEMIs), and is correlated with a poor prognosis. In the large-scale, multicenter, randomized Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, the direct thrombin inhibitor bivalirudin compared to unfractionated heparin (UFH) plus a glycoprotein IIb/IIIa inhibitor (GPI) significantly reduced major bleeding while affording comparable rates of composite ischemia in patients with acute STEMIs who underwent primary percutaneous coronary intervention (PCI), which resulted in significantly improved survival. Whether bivalirudin is most beneficial in patients with baseline anemia has not been evaluated. In addition, no studies have addressed in detail whether there are gender-specific differences in the outcomes of patients with anemia treated with primary PCI using contemporary antithrombotic therapies. We therefore examined the database from the HORIZONS-AMI trial to determine (1) the impact of anemia on the outcomes of patients with STEMIs treated with primary PCI in relation to contemporary adjunctive antithrombotic medications and (2) whether there are gender-specific differences in the outcomes of patients with anemia.
Methods
The HORIZONS-AMI protocol, inclusion and exclusion criteria, and principal results have been reported in detail elsewhere. Briefly, 3,602 patients aged ≥18 years who had persistent ST-segment elevation ≥1 mm in ≥2 contiguous leads, new left bundle branch block, or true posterior infarctions and symptoms consistent with acute myocardial infarctions lasting >20 minutes but <12 hours who underwent primary PCI were randomized 1:1 to treatment with bivalirudin alone (Angiomax; The Medicines Company, Parsippany, New Jersey) versus UFH plus a GPI (either abciximab or eptifibatide). Exclusion criteria were contraindications to the study medications, previous administration of thrombolytic agents, current use of warfarin, history of bleeding diathesis, stroke or transient ischemic attack within the previous 6 months or any permanent neurologic deficit, refusal to receive blood transfusions, gastrointestinal or genitourinary bleeding within the previous 2 months, major surgery within the previous 6 weeks, a known platelet count <100,000 cells/mm 3 or a hemoglobin level <10 g/dl (although baseline laboratory values did not have to be available before enrollment), planned elective surgery that would necessitate an interruption in treatment with thienopyridines during the first 6 months after enrollment, and coronary stent implantation within the previous 30 days. Immediate coronary angiography with left ventriculography was performed after randomization, followed by PCI, coronary artery bypass grafting, or medical management at the discretion of the physician. After patency was restored in the infarct-related vessel, eligible patients were randomly assigned again, in a 3:1 ratio, to either Taxus Express paclitaxel-eluting stents or visually indistinguishable Express bare-metal stents (Boston Scientific Corporation, Natick, Massachusetts).
Two primary 30-day end points were prespecified: major bleeding (not related to coronary artery bypass grafting) and combined adverse clinical events (consisting of major bleeding or a composite of major adverse cardiovascular events, including death, reinfarction, target vessel revascularization for ischemia, and stroke). Major bleeding was defined as intracranial or intraocular hemorrhage, a >5-cm hematoma at the access site, or bleeding requiring intervention, a decrease in the hemoglobin level ≥4 g/dl without an overt bleeding source or ≥3 g/dl with an overt bleeding source, reoperation for bleeding, or blood transfusion. Bleeding was also assessed and adjudicated on the basis of the Thrombolysis In Myocardial Infarction (TIMI) and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) scales. Minor bleeding did not meet the criteria of major bleeding. The component definitions of major adverse cardiovascular events have been previously described. Baseline anemia was defined using World Health Organization criteria as a hematocrit value at initial presentation <39% for men and <36% for women. Thrombocytopenia was the occurrence of a nadir in-hospital platelet count ≤150 × 10 9 /L. Thrombocytopenia was further subdivided into mild, moderate, severe, and profound (nadir platelets count 100 to <150 × 10 9 /L, 50 to <100 × 10 9 /L, 20 to <50 × 10 9 /L, and <20 × 10 9 /L, respectively). Renal insufficiency was defined as a creatinine clearance <60 ml/min estimated at baseline using the Cockcroft-Gault equation. Renal function deterioration was defined as a ≥25% increase in serum creatinine or an absolute increase ≥0.5 mg/dl compared to the baseline value. Stent thrombosis was defined as the definite or probable occurrence of a stent-related thrombotic event according to the Academic Research Consortium classification. All events were adjudicated by an independent clinical events committee blinded to treatment assignment.
Outcomes were stratified in relation to the presence or absence of anemia and gender. Categorical variables were compared using chi-square statistics or Fisher’s exact test. Continuous variables were compared using the Kruskal-Wallis test and are presented as medians with interquartile ranges. Survival data were estimated using the Kaplan-Meier method and were compared using the log-rank test. Multivariate analysis of predictors of clinical events at 1-year follow-up was performed using Cox proportional-hazards regression with stepwise selection using entry and exit criteria of p < 0.10. The candidate variables entered into the model included demographics (age, gender, and race), the presence or absence of co-morbidities (hypertension, hyperlipidemia, smoking, diabetes mellitus, peripheral vascular disease, and congestive heart failure), medical history (angina, PCI, myocardial infarction, and coronary artery bypass grafting), Killip class and body mass index on admission, baseline laboratory values (presence or absence of anemia, platelet count, white blood count, and creatinine clearance <60 ml/min), angiographic features (left anterior descending coronary artery infarct vessel, reference vessel diameter, target lesion length, and the left ventricular ejection fraction), and index hospitalization and procedural details (duration from symptom onset to first balloon inflation, antithrombotic randomization, clopidogrel loading dose, randomization to paclitaxel-eluting stent vs bare-metal stent, number of stents, use of heparin before randomization, and final TIMI flow grade 3). All analyses were 2 sided, and significance was established at the 0.05 level.
Results
Patient flow and follow-up appear in Figure 1 . Among a total of 3,602 patients in the HORIZONS-AMI trial, baseline hematocrit before angiography was available in 3,389 patients (94.1%), including 2,603 men (76.8%) and 786 women (23.2%). Primary PCI was attempted in a total of 3,153 of these patients (93.0%), including 2,441 of the men (77.4%) and 712 of the women (22.6%), who thus constituted the study population. From this group, anemia at baseline was present in 331 patients (10.5%), including 237 men (9.7%) and 94 women (13.2%).
Patients with versus without baseline anemia were older, were more commonly women, and more frequently had coexisting co-morbidities as well as histories of myocardial infarction and/or coronary artery bypass grafting ( Table 1 ). Smoking, however, was less prevalent in patients with versus without anemia. On admission, patients with versus without anemia more frequently had Killip class ≥2 and had lower values of hemoglobin, hematocrit, white blood cell count, and creatinine clearance. Principal angiographic and procedural data, rates of assignment to study drug, and randomization to paclitaxel-eluting stents or bare-metal stents did not differ significantly between the 2 groups ( Table 2 ).
| Variable | All Patients | Women (n = 712) | Men (n = 2,441) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Anemia | No Anemia | p Value ⁎ | Anemia | No Anemia | p Value ⁎ | Anemia | No Anemia | p Value ⁎ | |
| (n = 3,153) | (n = 331) | (n = 2,822) | (n = 94) | (n = 618) | (n = 237) | (n = 2,204) | ||||
| Clinical characteristics | ||||||||||
| Men | 77.4% | 71.6% | 78.1% | 0.008 | ||||||
| Age (years) | 60.0% | 67.7% | 59.2% | <0.0001 | 70.9% | 65.0% | 0.001 | 66.0% | 58.1% | <0.0001 |
| Age ≥65 years | 36.0% | 56.5% | 33.6% | <0.0001 | 64.9% | 49.8% | 0.007 | 53.2% | 29.0% | <0.0001 |
| Diabetes mellitus | 16.5% | 24.8% | 15.6% | <0.0001 | 27.7% | 18.1% | 0.03 | 23.6% | 14.8% | 0.0004 |
| Hypertension | 53.5% | 59.5% | 52.8% | 0.02 | 64.9% | 62.1% | 0.61 | 57.4% | 50.2% | 0.04 |
| Hyperlipidemia | 43.7% | 47.7% | 43.2% | 0.12 | 50.0% | 46.1% | 0.48 | 46.8% | 42.4% | 0.19 |
| Current smoking | 46.6% | 31.7% | 48.3% | <0.0001 | 26.6% | 44.2% | 0.001 | 33.8% | 49.5% | <0.0001 |
| Previous myocardial infarction | 10.5% | 15.1% | 9.9% | 0.004 | 10.6% | 6.5% | 0.14 | 16.9% | 10.9% | 0.006 |
| Previous coronary angioplasty | 10.6% | 16.4% | 10.0% | 0.0004 | 10.8% | 7.4% | 0.27 | 18.6% | 10.7% | 0.0003 |
| Previous coronary grafting | 2.7% | 4.2% | 2.5% | 0.07 | 5.3% | 1.1% | 0.01 | 3.8% | 2.9% | 0.44 |
| Peripheral arterial disease | 4.3% | 7.6% | 4.0% | 0.003 | 7.4% | 3.6% | 0.09 | 7.6% | 4.1% | 0.01 |
| Killip class ≥2 | 8.8% | 13.3% | 8.3% | 0.003 | 20.2% | 9.5% | 0.002 | 10.5% | 8.0% | 0.17 |
| Body mass index (kg/m 2 ) | 27.1 | 25.8 | 27.3 | <0.0001 | 25.3 | 27.2 | 0.007 | 25.9 | 27.3 | <0.0001 |
| Hemoglobin (g/dl) | ||||||||||
| Baseline | 14.6 | 12.1 | 14.8 | <0.0001 | 11.3 | 13.7 | <0.0001 | 12.5 | 15.1 | <0.0001 |
| Nadir | 13.0 | 11.1 | 13.1 | <0.0001 | 9,8 | 11.9 | <0.0001 | 11.7 | 13.4 | <0.0001 |
| Hematocrit (%) | ||||||||||
| Baseline | 43.0 | 35.0 | 43.0 | <0.0001 | 34.0 | 41.0 | <0.0001 | 37.0 | 44.0 | <0.0001 |
| Nadir | 38.0 | 32.0 | 39.0 | <0.0001 | 29.0 | 35.0 | <0.0001 | 34.0 | 39.0 | <0.0001 |
| White blood cell count (×10 9 /L) | 10. | 9.9 | 11.0 | <0.0001 | 10.7 | 11.1 | 0.12 | 9.8 | 11.0 | <0.0001 |
| Platelet count (×10 9 /L) | 247 | 244 | 248 | 0.12 | 262 | 269 | 0.36 | 235 | 241 | 0.06 |
| Serum creatinine (mg/dl) | 1.0 | 1.0 | 1.0 | 0.0004 | 1.0 | 0.8 | <0.0001 | 1.0 | 1.0 | 0.03 |
| Creatinine clearance <60 ml/min | 16.1% | 35.6% | 13.8% | <0.0001 | 55.6% | 27.2% | <0.0001 | 27.5% | 10.1% | <0.0001 |
| Randomization to antithrombotic therapy | ||||||||||
| Bivalirudin | 50.3% | 50.2% | 50.3% | 0.97 | 50.0% | 48.7% | 0.82 | 50.2% | 50.7% | 0.88 |
| UFH + GPI | 49.7% | 49.8% | 49.7% | 0.97 | 50.0% | 51.3% | 0.82 | 49.8% | 49.3% | 0.88 |
⁎ Anemic versus not anemic for all patients and respective gender.
| Variable | All Patients | Women (n = 712) | Men (n = 2,441) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Anemia | No Anemia | p Value ⁎ | Anemia | No Anemia | p Value ⁎ | Anemia | No Anemia | p Value ⁎ | |
| (n = 3,153) | (n = 331) | (n = 2,822) | (n = 94) | (n = 2,822) | (n = 237) | (n = 2,822) | ||||
| Angiographic findings | ||||||||||
| LVEF site reported (%) | 50 | 50 | 50 | 0.49 | 50 | 50 | 0.53 | 50 | 50 | 0.64 |
| Infarct-related artery | ||||||||||
| Left anterior descending | 40% | 37% | 40% | 0.22 | 32% | 40% | 0.15 | 39% | 41% | 0.57 |
| Left circumflex | 15% | 12% | 15% | 0.04 | 11% | 14% | 0.39 | 12% | 16% | 0.07 |
| Right | 44% | 49% | 43% | 0.03 | 55% | 46% | 0.09 | 47% | 43% | 0.16 |
| Saphenous vein graft | 1% | 2% | 1% | 0.02 | 2% | 0% | 0.133 | 2% | 1% | 0.04 |
| Baseline lesion length (mm) | 14.60 | 15.00 | 14.50 | 0.33 | 13.51 | 14.22 | 0.45 | 15.00 | 14.62 | 0.10 |
| Final RVD (mm) | 2.91 | 2.92 | 2.91 | 0.45 | 2.82 | 2.76 | 0.18 | 2.95 | 2.95 | 0.57 |
| Final TIMI grade 3 flow | 87% | 86% | 87% | 0.89 | 88% | 86% | 0.57 | 86% | 87% | 0.62 |
| Final CTFC | 20.00 | 20.00 | 20.00 | 0.79 | 19.50 | 20.00 | 0.82 | 20.00 | 20.00 | 0.97 |
| Final MBG 3 | 86% | 83% | 86% | 0.14 | 83% | 86% | 0.41 | 83% | 87% | 0.22 |
| Final MLD (mm) | 2.33 | 2.33 | 2.32 | 0.74 | 2.23 | 2.20 | 0.66 | 2.39 | 2.36 | 0.58 |
| Final diameter stenosis (%) | 18.7 | 18.7 | 18.7 | 0.90 | 19.6 | 19.2 | 0.57 | 18.2 | 18.6 | 0.74 |
| Procedural data | ||||||||||
| Symptom onset to balloon (hours) | 3.7 | 3.8 | 3.7 | 0.35 | 4.1 | 4.0 | 0.83 | 3.7 | 3.6 | 0.44 |
| Door to balloon (hours) | 1.7 | 1.6 | 1.7 | 0.65 | 1.6 | 1.7 | 0.29 | 1.6 | 1.6 | 0.95 |
| Total stent length (mm) | 24.0 | 24.0 | 24.0 | 0.17 | 28.0 | 24.0 | 0.60 | 24.0 | 24.0 | 0.19 |
| Aspiration catheter use | 11.4% | 14.5% | 11.1% | 0.07 | 13.8% | 9.7% | 0.21 | 14.7% | 11.5% | 0.15 |
| Distal protection device | 0.5% | 0.6% | 0.5% | 0.70 | 0.0% | 0.0% | NA | 0.9% | 0.7% | 0.68 |
| Use of GPI | 55.8% | 56.5% | 55.7% | 0.79 | 58.5% | 56.1% | 0.67 | 55.7% | 55.6% | 0.98 |
| GPI with bivalirudin (bailout) | 6.2% | 6.6% | 6.2% | 0.73 | 8.5% | 5.3% | 0.22 | 5.9% | 6.4% | 0.77 |
| Peak ACT time (seconds) | 310.0 | 310.0 | 310.0 | 0.72 | 330.0 | 316.0 | 0.93 | 307.0 | 310.0 | 0.63 |
| Total amount of contrast (ml) | 230.0 | 230.0 | 230.0 | 0.54 | 210.0 | 220.0 | 0.59 | 240.0 | 230.0 | 0.65 |
| Stent randomization assignment | ||||||||||
| Paclitaxel-eluting stent | 74.9% | 81.3% | 74.1% | 0.008 | 80.5% | 73.3% | 0.15 | 81.7% | 74.4% | 0.02 |
| Bare-metal stent | 25.1% | 18.7% | 25.9% | 0.008 | 19.5% | 26.7% | 0.15 | 18.3% | 25.6% | 0.02 |
| Concomitant medications during index hospitalization | ||||||||||
| Aspirin | 99.8% | 99.7% | 99.9% | 0.43 | 100.0% | 99.8% | 1.0 | 99.6% | 99.9% | 0.34 |
| Clopidogrel | 99.5% | 99.1% | 99.5% | 0.23 | 98.9% | 99.7% | 0.35 | 99.2% | 99.5% | 0.36 |
| Loading dose of 600 mg | 63.3% | 64.0% | 63.2% | 0.78 | 60.9% | 61.0% | 0.98 | 65.2% | 63.8% | 0.67 |
⁎ Anemic versus not anemic for all patients and respective gender.
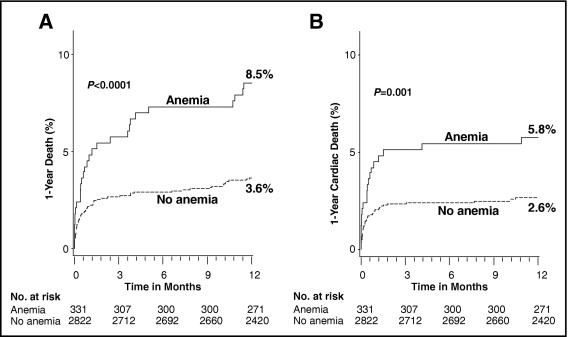
As seen in Figure 2 and listed in Table 3 , patients with versus without baseline anemia had significantly higher rates of 30-day and 1-year all-cause mortality and cardiac mortality, as well as higher 30-day rates of stroke, with a similar trend at 1 year. The occurrence of reinfarction, target vessel revascularization, and stent thrombosis was not related to baseline anemia.
| Outcomes | Anemia | No Anemia | p Value |
|---|---|---|---|
| (n = 331) | (n = 2,822) | ||
| All-cause mortality | |||
| 30-day | 4.8% | 2.2% | 0.003 |
| 1-year | 8.5% | 3.6% | <0.0001 |
| Cardiac mortality | |||
| 30-day | 4.5% | 2.0% | 0.004 |
| 1-year | 5.8% | 2.6% | 0.001 |
| Reinfarction | |||
| 30-day | 1.9% | 2.0% | 0.81 |
| 1-year | 4.5% | 4.2% | 0.84 |
| Target vessel revascularization for ischemia | |||
| 30-day | 2.5% | 2.4% | 0.92 |
| 1-year | 8.4% | 6.9% | 0.35 |
| Stroke | |||
| 30-day | 1.5% | 0.4% | 0.006 |
| 1-year | 1.9% | 0.8% | 0.06 |
| Protocol-defined major bleeding (non-CABG-related) | |||
| 30-day | 13.5% | 6.7% | <0.0001 |
| 1-year | 14.8% | 7.2% | <0.0001 |
| Protocol-defined minor bleeding | |||
| 30-day | 14.8% | 11.6% | 0.09 |
| Life-threatening/severe/moderate GUSTO bleeding | |||
| 30-day | 12.3% | 3.0% | <0.0001 |
| 1-year | 13.2% | 3.4% | <0.0001 |
| TIMI major bleeding | |||
| 30-day | 4.0% | 3.5% | 0.62 |
| 1-year | 4.6% | 3.7% | 0.42 |
| TIMI minor bleeding | |||
| 30-day | 4.9% | 3.7% | 0.28 |
| 1-year | 5.2% | 3.8% | 0.21 |
| Blood transfusions | |||
| 30-day | 11.4% | 2.2% | <0.0001 |
| 1-year | 12.4% | 2.5% | <0.0001 |
| Major adverse cardiovascular events | |||
| 30-day | 8.2% | 5.0% | 0.02 |
| 1-year | 18.4% | 11.5% | 0.0003 |
| Net adverse clinical events | |||
| 30-day | 19.3% | 10.3% | <0.0001 |
| 1-year | 28.3% | 16.2% | <0.0001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


