Fig. 15.1
A 61-year-old female with DM-2 and no cardiac complaints. Myocardial perfusion scintigraphy showed normal perfusion at rest and during exercise. Planar cardiac [123I]-MIBG scintigraphy at 15 min (i.e., early) and 4 h (i.e., late) postinjection (left and right panels). The early and late [123I]-MIBG H/M ratios are to be considered as normal. The calculated myocardial washout of [123I]-MIBG was approximately 6 % (also within the normal range). However, the reconstructed cardiac [123I]-MIBG SPECT images clearly showed reduced [123I]-MIBG uptake in the inferior myocardial wall
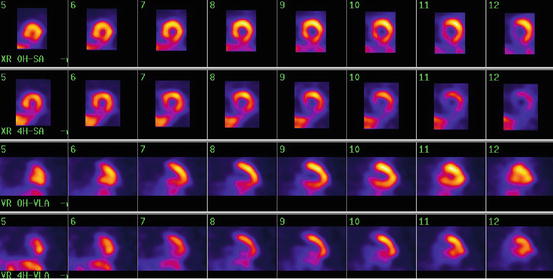
Fig. 15.2
Early and delayed short-axis (SA) and vertical long-axis (VLA) views
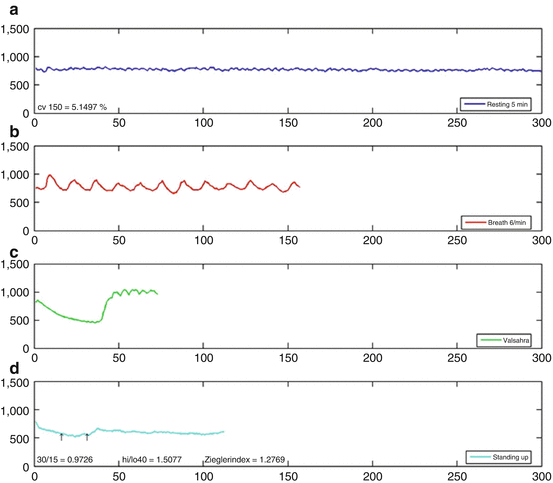
Fig. 15.3
Normal HRV registration measurement of the same patient as depicted in Fig. 15.2. This nicely illustrates that cardiac [123I]-MIBG seems to be more sensitive for detecting possible cardiac autonomic neuropathy. (a) Beat-to-beat variation during 5 min at rest. (b) RR interval during 2 min of deep inspiration and expiration. (c) RR interval during Valsalva maneuver of 15 s. (d) RR interval during laying to standing test
While HRV and other traditional parameters provide an impression of global innervation abnormalities, [123I]-MIBG scintigraphy with SPECT provides information on regional innervation. The findings in the study from Scholte indicated that regional abnormalities occur often in patients with asymptomatic diabetes. Other studies using [123I]-MIBG scintigraphy, in populations with varying cardiovascular diseases, have also shown regional innervation abnormalities (Schnell et al. 1995, 1996, 2002; Scott and Kench 2004). For example, Langer and colleagues, evaluated 65 diabetic patients and noted significantly impaired [123I]-MIBG uptake in the inferior wall and apex (Langer et al. 1995). Additional studies have shown that abnormalities in CAN tend to occur first in the inferior regions of the myocardium and then progressively spread to adjacent segments (Schnell et al. 1997; Hattori et al. 1996).
15.5.2 Positron Emission Tomography
Absolute quantification of myocardial sympathetic innervation is possible with PET imaging. Using carbon-11 metahydroxyephedrine ([11C]-mHED) in PET imaging has the advantage of accurately detecting regional abnormalities in sympathetic innervation. Regional abnormalities in cardiac sympathetic innervation with [11C]-mHED PET imaging in 29 diabetic patients compared with ten healthy subjects has been studied by Stevens and coworkers (Stevens et al. 1998). The diabetic patients were categorized into the presence of mild or severe diabetic autonomic neuropathy. Using the absolute difference in myocardial tracer uptake, the extent of regional sympathetic denervation was expressed as the percentage of the left ventricle (LV) in all subjects with diabetes. The study showed that the extent of regional sympathetic denervation was significantly larger in patients with severe autonomic neuropathy compared with patients with mild autonomic neuropathy (48 ± 19 % vs. 6 ± 5 %, P < 0.01). Furthermore, there was evidence of sympathetic dysinnervation with increased innervation in the basal myocardial segments but decreased innervation in the apical myocardial segments. This variation in regional myocardial variation in sympathetic innervation could contribute to myocardial electrical instability and potentially life-threatening arrhythmias.
15.6 DM CAN in Heart Failure Patients
The role of [123I]-MIBG scintigraphy in predicting heart failure (HF) progression in patients with and without DM has been worked out in a substudy of the ADMIRE-HF study (Gerson et al. 2011; Jacobson et al. 2010). In this international multicenter study, the prognostic value of [123I]-MIBG cardiac imaging in 961 subjects with NYHA class II-III heart failure and left ventricular ejection fraction (LVEF) < 35 % has been evaluated. Progression of HF, defined as an increase in NYHA class (from II to III/IV or from III to IV), was observed in 22 % of patients with DM compared to 14 % in those without DM (P = 0.005). In patients with DM, the late H/M ratio was lower than in those without DM, and only 21 % of patients with DM in the study had a late H/M ratio ≥ 1.6. This is consistent with cardiac autonomic neuropathy in patients with HF in general, regardless of the presence of DM. Moreover, in heart failure patients with H/M ratio < 1.6, DM was associated with an three times increased risk of HF progression over 2 years compared to those with DM with normal [123I]-MIBG uptake (RR, 2.99; P < 0.001). The late H/M ratio was an independent, incremental predictor of HF progression in addition to B-type natriuretic peptide, LVEF, and NYHA class, supporting the association of DM with alterations in cardiac structure and function and highlighting the contribution of cardiac autonomic denervation to the development and progression of HF (Boudina and Abel 2007).
As discussed by Gerson and colleagues, different pathways may be responsible for this observation (Gerson et al. 2011). A defective angina warning system can interfere with identification of myocardial ischemia and infarction (i.e., silent) preventing application of effective therapies for ischemic heart disease. In addition, this defective warning system leads to an activation of the renin-angiotensin-aldosterone system, tachycardia by uninhibited sympathetic activity, orthostatic hypotension, and uncontrolled hypertension (Gerson et al. 2011). As a result, left ventricular systolic and diastolic dysfunction can progress. Also, autonomic dysfunction involving cardiac efferent sympathetic nerve transmission is an important determinant of coronary blood flow under conditions of increased sympathetic stimulation. In response to sympathetic activation by cold pressor stress, Di Carli and colleagues showed a 31 ± 12 % increase in myocardial blood flow with a 13 % fall in coronary vascular resistance in diabetics without sympathetic nerve dysfunction compared to only a 14 ± 10 % increase in myocardial blood flow and a 5 % increase in coronary resistance in diabetics with sympathetic nerve dysfunction (Di Carli et al. 1999).
15.7 Clinical Implications and Conclusions
Of all established diabetes mellitus-related and cardiac risk factors in patients with diabetes, poor glycemic control is of great importance in the development and progression of CAN (Ziegler et al. 1998; Schnell et al. 1997). Accordingly, early detection of CAN is of utmost importance, and cardiac [123I]-MIBG scintigraphy appears more sensitive than HRV to detect CAN in diabetic patients. Identification of these patients may permit risk factor modification, and intensive medical treatment, aiming at better glycemic control, which in turn may favorably affect outcome (Anan et al. 2005, 2006, 2007a, b). Indeed, several studies have shown that improvement of CAN (as evidenced by HRV) can be achieved by weight loss as a result of regular exercise (Howorka et al. 1997; Kontopoulos et al. 1997). Furthermore, progression of CAN can also be delayed by intensive medical therapy, thereby reducing the risk of premature mortality and progression of heart failure (Ziegler 1994).
References
American Diabetes Association (1998) Consensus development conference on the diagnosis of coronary heart disease in people with diabetes: 10–11 February 1998, Miami, Florida. Diabetes Care 21:1551–1559CrossRef
American Diabetes Association (2008) Diagnosis and classification of diabetes mellitus. Diabetes Care 31:S55–S60CrossRef
Anan F, Masaki T, Yonemochi H et al (2007a) Abdominal visceral fat accumulation is associated with the results of (123)I-metaiodobenzylguanidine myocardial scintigraphy in type 2 diabetic patients. Eur J Nucl Med Mol Imaging 34:1189–1197PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


