Fig. 19.1
Delayed [123I]-MIBG images before (a, c) and after (b, d) CRT. Responder patient images with a HMR that improved from 1.66 to 2.01 (a, b). Non-responder patient images with a HMR that showed no difference from 0.99 to 0.98 (c, d)
Likewise Burri et al. observed that responders to ≥6 months of CRT showed lower [123I]-MIBG WR at follow-up when compared with non-responders, indicating improvement of cardiac sympathetic nerve activity (Burri et al. 2008). The decrease in WR at follow-up was only seen in the responders and paralleled an improvement in LVEF. Subsequently, Shinohara et al. found that late HMR significantly increased 6 months after CRT in responders but not in the non-responders (Shinohara et al. 2011). Furthermore, Cha et al. reported in a prospective study that included 45 consecutive HF patients on CRT that responders (22 patients) had a significantly higher late HMR (2.11 vs. 1.48) and lower WR (37 % vs. 62 %) at baseline than non-responders (Cha et al. 2011). Recently, Tanaka et al. found that HF patients with dyssynchrony had significantly less cardiac sympathetic activity than those without dyssynchrony (late HMR 1.62 ± 0.31 vs. 1.82 ± 0.36) despite having similar LVEF (Tanaka et al. 2012). Dyssynchrony and late HMR ≥1.6 were associated with a high frequency of response to CRT and favorable long-term outcome over 3 years. In a recent systematic review, Scholtens et al. analyzed the available evidence regarding cardiac [123I]-MIBG imaging and CRT application. Nine studies were selected from this search, including in total 225 patients. Regardless of the accounted differences between the included studies, a correlation between improvement of cardiac innervation parameters and response to CRT was evident. Improvement of sympathetic innervations was found in patients who responded to CRT. This also strengthens the notion that diminished late HMR is related to severe heart failure without response to CRT. Nevertheless, the authors recognize the limitations in availability of data and the need for further research, standardization of the techniques, and integration of the method with other diagnostic parameters (Scholtens et al. 2013).
Ventricular assist devices for mechanical circulatory support may be used as a “bridge to decision” or longer term in selected patients presenting with end-stage HF (McMurray et al. 2012). Drakos et al. recently reported that LV assist device therapy caused clinical, functional, and hemodynamic improvements accompanied by improvements in [123I]-MIBG imaging (Drakos et al. 2010). These observations might have important implications, particularly for recipients of ventricular assist devices whose native cardiac function is difficult to evaluate. Improvements in [123I]-MIBG imaging could potentially identify responders to mechanical circulatory support who might become candidates for explanation of the device after the development of a potentially sustained myocardial functional recovery. Further studies are needed to determine if patients with severe HF and a poor versus favorable cardiac adrenergic imaging to maximal medical therapy have a more favorable outcome with ventricular assist devices compared to continued medical therapy alone.
19.6 PET Cardiac Innervation Studies
[11C]-mHED PET is the most commonly used tracer for the assessment of cardiac sympathetic innervation activity. PET has demonstrated superior results to [123I]-MIBG SPECT regarding resolution and homogeneity of tracer distribution (Bengel et al. 2009). Also PET is able to image more details by higher spatial resolution and higher signal-to-noise ratio, resulting in better regional abnormality analysis (Matsunari et al. 2010). Finally, absolute quantification by using kinetic modeling provides more accurate quantification in comparison with [123I]-MIBG SPECT imaging.
An important advance in the last decade has been the organization of a first large-scale PET clinical trial (PAREPET) evaluating the viability of autonomic neuronal PET in prediction of arrhythmia (Fallavollita et al. 2006, 2014). This study included prospectively 204 patients with ischemic cardiomyopathy who were eligible for an ICD as primary prevention. [11C]-mHED, [13N]NH3, and [18F]-FDG PET scans were performed, and all patients were followed for sudden cardiac arrest (SCA) primarily. Interestingly, the results emphasized the importance of regional quantification of the sympathetic neuronal function. The authors concluded that sympathetic denervation predicts SCA mortality, independently from LVEF and infarct volume (Fig. 19.2). Implantation of CRT device also needs to be adapted per patient. Selection of patients that will benefit from CRT implantation is mandatory. Not all patients will experience improvement of cardiac complaints and LVEF after CRT implantation. A relationship between CRT response and effect on peripheral sympathetic nerve activity has been described for [11C]-mHED in a first study of Noordzij and coworkers (Noordzij et al. 2014). In seven heart failure patients, [11C]-mHED was used for the evaluation of cardiac sympathetic innervation, before and 6 months after the treatment with cardiac resynchronization therapy (CRT). All seven patients were responders based on echocardiographic parameters (especially decrease in end-diastolic volume >10 %). Two patients showed an increase in [11C]-mHED uptake after CRT implantation. Both patients also showed a significant increase in LVEF, determined with multiple gated acquisition (MUGA). The other five patients did not show a change in [11C]-mHED uptake or LVEF, although they were responders to CRT (Fig. 19.3).
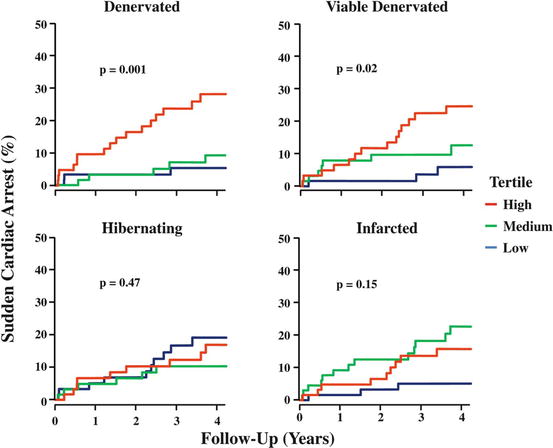
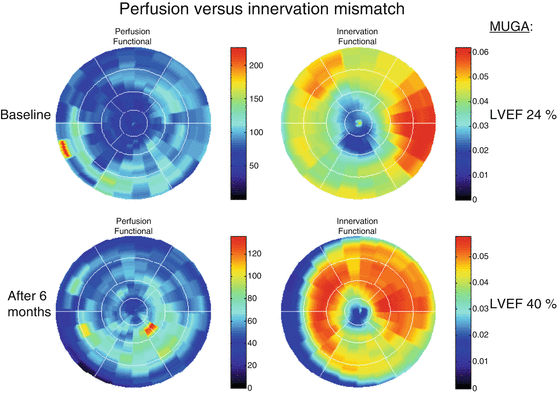

Fig. 19.2
Kaplan-Meier curves showing significantly lower survival in patients with large denervation, also in comparison with myocardial viability ([18F]-FDG) and left ventricular wall hibernation (From Fallavollita et al. (2014))

Fig. 19.3
Left polar maps indicate myocardial perfusion ([13N]NH3); right polar maps indicate sympathetic innervation ([11C]-mHED). Patient who showed response to the CRT scanned after 6 months. Mean [11C]-mHED increased from 0.035 to 0.041 mL/min/mL after CRT application, combined with improvement of LVEF (Noordzij et al. 2014)
At the same time, imaging sympathetic innervations with PET have been useful in assessing neuronal reinnervation in patients who undergo orthotopic heart transplantation (Bengel et al. 2001a, b) (Schwaiger et al. 1991). In heart-transplant recipients, the restoration of sympathetic innervations imaged with [11C]-mHED PET is associated with improved responses of the heart rate and contractile function to exercise. These results supported the functional importance of reinnervation in transplanted hearts. Mäki and coworkers studied the effect of bone marrow cell (BMC) therapy in 19 patients with acute ST-segment elevation myocardial infarct (STEMI) in a double-blind (including placebo) multicenter study (Mäki et al. 2012). They evaluated the feasibility of serial [11C]-mHED and [18F]-FDG PET and MRI studies to get more insight into the effects of BMCs on the healing process of ischemic myocardial damage. There was a decrease in [11C]-mHED defect size (−4.9 ± 4.0 vs. −1.6 ± 2.2 %, p = 0.08) and an increase in [18F]-FDG uptake in the infarct area at risk (0.06 ± 0.09 vs. −0.05 ± 0.16, p = 0.07) compared to controls, as well as less left ventricular dilatation (−4.4 ± 13.3 vs. 8.0 ± 16.7 mL/m2, p = 0.12) at 6-month follow-up. However, BMC treatment was inferior to placebo in terms of changes in rest perfusion in the area at risk (−0.09 ± 0.17 vs. 0.10 ± 0.17, p = 0.03) and infarct size (0.4 ± 4.2 vs. −5.1 ± 5.9 g, p = 0.047), and no effect was observed on ejection fraction (p = 0.37). It was concluded that after acute phase of STEMI, BMC therapy showed only minor trends of long-term benefit in patients with rapid successful thrombolysis.
19.7 Conclusion
Cardiac autonomic innervation represents a viable target for nuclear assessment using SPECT and PET techniques. Cardiac innervation is especially susceptible to chronic and sometime subclinical damage in a variety of conditions including HF, CAD, DCM, and DM. Several SPECT and PET radiotracers have been developed to optimize and improve autonomic innervation evaluation. Still [123I]-MIBG SPECT is the major player in the clinical field.
The use of cardiac adrenergic imaging may have value to risk stratify patients with HF and guided therapies. Selection of patients for ICD or CRT implantation is an important application of sympathetic innervation imaging with PET and SPECT and will possibly be cost-effective to explore.
There are many reports demonstrating that cardiac adrenergic imaging effectively monitors the effects of conventional HF medical therapies. The benefits of treatments that ameliorate the effects of neurohumoral imbalance in patients with HF are well established. As clinical status and/or LV function improves in response to β-blockers, ACEI or ARB, and MRA, there is a parallel improvement in cardiac sympathetic nerve function as assessed by radionuclide imaging. There is currently no potential advantage of cardiac adrenergic imaging to monitor the response to proven therapy in patients who respond well to conventional HF therapy with full therapeutic doses.
Cardiac adrenergic imaging might instead be more useful as an indicator of whether or not a patient’s medical therapy and dosages used are effective and could therefore help determine whether higher-risk and usually more expensive device therapies or cardiac transplantation is needed.
Ongoing development and clinical trials will probably clarify PET scanning advantages as well as new tracer applications and performance including not only sympathetic neurons (Nishijima et al. 2000) but also with a new focus on receptors and parasympathetic targets.
References
Agostini D, Belin A, Amar MH et al (2000) Improvement of cardiac neuronal function after carvedilol treatment in dilated cardiomyopathy: a 123I-MIBG scintigraphic study. J Nucl Med 41:845–851PubMed
Bengel FM, Higuchi T, Javadi MS et al (2009) Cardiac positron emission tomography. J Am Coll Cardiol 54:1–15PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


