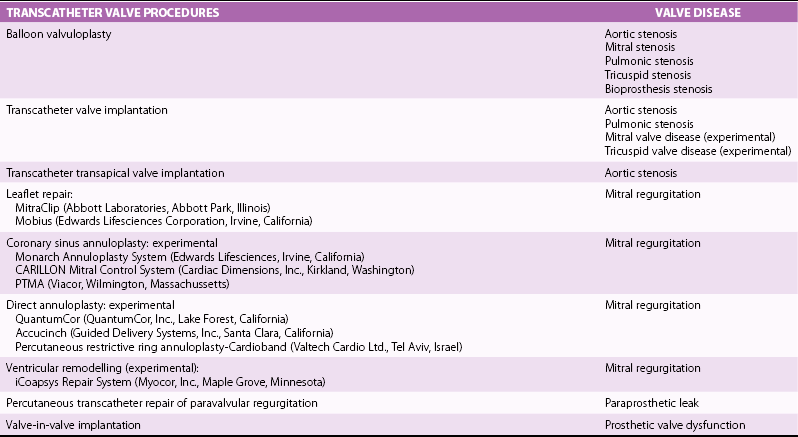
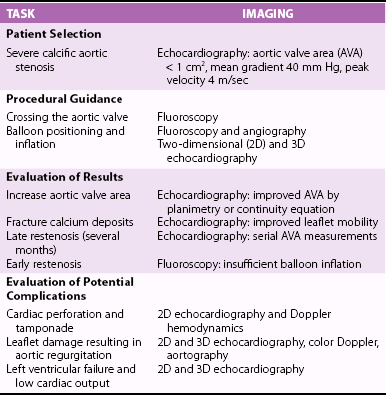
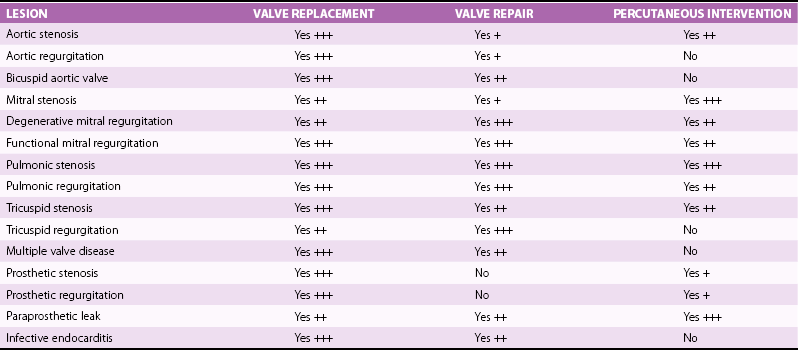
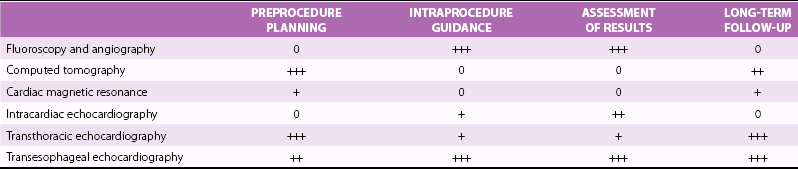
Chapter 16 VALVULAR HEART DISEASE IN THE ERA OF TRANSCATHETER VALVE PROCEDURES APPROACHES TO IMAGING GUIDANCE TRANSCATHETER TREATMENT OF VALVE DISEASE TRANSCATHETER BALLOON VALVE PROCEDURES TRANSCATHETER VALVE IMPLANTATION TRANSCATHETER PROCEDURES FOR MITRAL REGURGITATION TRANSCATHETER REPAIR OF PARAVALVULAR REGURGITATION In population-based studies the overall age-adjusted prevalence of valvular disease has been estimated to be 2.5% of the population. The prevalence according to the type of valvular disease is 1.7% for mitral regurgitation, 0.5% for aortic regurgitation, 0.4% for aortic stenosis, and 0.1% for mitral stenosis. Importantly, and relevant to this chapter, the prevalence of valvular heart disease increases significantly with age, from less than 2% in persons younger than 65 years, to 8.5% of those between 65 and 75 years, to 13.2% of those older than 75 years. 1 Although rheumatic heart disease remains common in developing countries, where its prevalence is estimated at 2% to 3%, the etiology and epidemiology of valvular heart disease have shifted in industrialized countries from rheumatic heart disease, a disease of the young, to calcific valve disease, a disease of the elderly.2,3 The decrease in the prevalence of rheumatic heart disease has been outweighed by an increase in age-related valve diseases.2,4,5 Calcific valve disease is characterized by the formation of cartilaginous deposits in the mitral valves and bone formation in the calcified aortic valves; 6 the main characteristic of this valvular pathology is an increase in its prevalence with age (see Chapters 3 and 4). In addition to aortic stenosis, mitral regurgitation is increasingly common in the elderly due to age-related degenerative changes of the valve leaflets or due to secondary mitral regurgitation with ischemic or myocardial disease. 7 Furthermore, given the increase in life expectancy, the burden of valvular disease in the elderly is expected to rise. Over the past decade, valvular procedures have increased not only in number (accounting now for more than 20% of all cardiac surgeries) but also in complexity. A third of patients referred for the management of valvular disease have previously undergone open heart surgery. 5 Comorbidities and frailty in the elderly significantly raise surgical morbidity and mortality, 8 so alternatives to surgery have been developed to provide less invasive forms of therapy in this aging population. Guidelines from Europe 9 and the United States 10 for the management of valvular heart disease now include recommendations for transcatheter valve procedures. The high level of interest in transcatheter valve procedures is apparent from the number of new publications and reviews that address this topic.11,12 Tables 16-1 and 16-2 summarize the established mechanical interventions used in advanced valvular heart disease. TABLE 16-1 Established Mechanical Interventions in Valvular Heart Disease Yes, Used; No, not used; +++, favored use; ++, common use; +, occasional use. The interventional field has moved forward not only in the invention of new devices but also because of breakthroughs, refinements, and implementation of imaging guidance that enable the interventionalist to perform these new treatments. 13 Cardiovascular imaging, including fluoroscopy and angiography, computed tomography (CT), cardiac magnetic resonance (CMR) imaging, and echocardiography, have played a central role in the development of transcatheter valve procedures. 14 Periprocedural imaging for patient selection and procedural planning and guidance are critical for the success of a structural heart disease program and constitute one of the cornerstones for the new paradigm in the management of valvular heart disease by a multidisciplinary heart team.15,16 Fluoroscopy, angiography, and two-dimensional echocardiography maintain a central role in procedural guidance. Novel three-dimensional (3D) imaging modalities are gaining importance; these include CT, CMR, 3D echocardiography, and rotational angiography.17,18 The choice of imaging guidance modality is based on specific differences between imaging systems as well as other variables, such as cost, clinical scenario, operator expertise, and complexity of procedure. 19 The roles and relative values of different cardiac imaging modalities in common transcatheter valve procedures are outlined on Tables 16-3 And 16-4. TABLE 16-3 Value of Imaging Modalities in Valvular Heart Disease Interventions 0, No value; +, little value; ++, moderate value; +++, best value. TABLE 16-4 Role and Relative Value of Different Cardiac Imaging Modalities on Common Transcatheter Valve Procedures +, Little value, ++, mild value; +++, moderate value; ++++, most value. Fluoroscopy and cineangiography have traditionally been used in the catheterization laboratory to guide coronary interventional procedures; today they are the central guidance tools for noncoronary interventions. 14 Because only radiopaque objects are visible with these modalities, radiopaque dye has to be injected to visualize chambers, great vessels, and outlines of valves; nevertheless, fluoroscopy is an integral part of all cardiac catheterization laboratories, and all of the catheters and devices are radiopaque, having been engineered for their use and deployment in the catheterization laboratory. The major advantages of image guidance with fluoroscopy and cineangiography are well exemplified in patients undergoing transcatheter aortic valve replacement, as illustrated in Figure 16-1. The major disadvantages of fluoroscopy and cineangiography include radiation exposure, lack of visualization of the myocardium and valvular tissue, and the need to inject radiopaque and potentially nephrotoxic contrast agents. FIGURE 16-1 Fluoroscopy and angiography in guidance of transcatheter aortic valve implantation. New radiography-based imaging techniques under development, referred to as three-dimensional rotational angiography and C-arm CT, hold great promise for improving current device implantation and the understanding of cardiovascular anatomy. A variety of anatomic targets, from the aortic root and pulmonary arteries (for percutaneous aortic and pulmonic valve implantation) to general visualization and assessment of the whole heart, are being explored20,21 ( Figure 16-2A). Fluoroscopy has been fused to 3D transesophageal echocardiography (TEE), providing simultaneous imaging with both techniques and allowing for similar anatomic perspective ( Figure 16-2B). FIGURE 16-2 Next-generation image guidance systems for valvular interventions. Multidetector CT (MDCT) provides 3D volumetric data sets, allowing multiplane reconstructions of the heart and great vessels. It plays an important role in preprocedural screening for and planning of several transcatheter valve interventions, thereby improving procedural outcomes and minimizing procedure-related complications.22,23 In patients being considered for percutaneous transcatheter aortic valve implantation (TAVI), MDCT can provide detailed information on the shape and size of the aortic annulus and the relation between the annulus and the ostia of the coronary arteries24–26 ( Figures 16-3 and 16-4). FIGURE 16-3 Cardiac computed tomography before and after transcatheter aortic valve implantation. FIGURE 16-4 Cardiac computed tomography key measurements for transcatheter aortic valve implantation. Aortic valve calcification as assessed by MDCT has been shown to be well correlated to aortic valve area (AVA) and may be a useful adjunct for the evaluation of the severity of aortic stenosis severity, especially in difficult cases such as patients with low ejection fraction (EF). 27 As described later, MDCT enables an accurate sizing of the aortic valve annulus and constitutes a valuable imaging tool to evaluate prosthesis location and deployment for TAVI. MDCT also facilitates the understanding of the underlying mechanisms of postprocedural aortic regurgitation. 28 In addition, preprocedural MDCT can be used to predict optimal angiographic deployment projections for implantation of transcatheter valves. 29 In patients being considered for TAVI, MDCT is used as the “gold standard” for determining of the appropriateness of the peripheral vasculature for percutaneous femoral access ( Figure 16-5). FIGURE 16-5 Digital angiography and computed tomography angiography for assessment of vascular access before transcatheter aortic valve implantation. Cardiac magnetic resonance (CMR) is a comprehensive noninvasive tool capable of evaluating all aspects of valvular heart disease. Its main advantages include direct quantification of regurgitant lesions, accurate assessment of ventricular size mass and function, and visualization of myocardial scar. 30 A system for x-ray fused with magnetic resonance imaging, has been developed and validated; this will be of value to guide catheter procedures with high spatial precision. 31 Interventional magnetic resonance imaging to guide procedures is evolving slowly, however, because it requires considerable capital investment and special compatible instruments. 14 Patients are imaged in the CMR imaging suite and then transported to fluoroscopic laboratories capable of some type of image fusion. CMR has been used to guide transcatheter valve implantation in the aortic valve position in experimental animals. CMR enables assessment of cardiovascular anatomy and function. During and after implantation, the position and function of the prosthetic valve is easy to determine. In addition, CMR provides immediate post-intervention physiologic parameters of cardiac function and proximal coronary artery perfusion. 32 Use of CMR to assess the aortic valve area and aortic root dimensions has been compared with a multimodality imaging approach including Doppler ultrasonography, 2-dimensional (2D) transthoracic echocardiography (TTE), 3D TTE and TEE, and catheterization. CMR and TEE provided similar assessments of the aortic valve annulus dimensions, especially at the limits of the TAVI range. 33 Intracardiac echocardiography (ICE) has been used for septal defect closure but also may be used for some valvular interventions.34–39 As seen in Figure 16-6, all four cardiac valves can be assessed with ICE, and the clarity of the structures is as good as and sometimes better than that with TEE. Yet because of the 2D aspect of ICE images, it is generally not used for interventional treatments such as MitraClip insertion (Abbott Laboratories, Abbott Park, Illinois) and TAVI. The tip of the ICE catheter must be placed in a location to allow accurate Doppler assessment of valvular lesions, both stenotic and regurgitant. ICE is expected to improve and to be better integrated into the procedure room. Unlike with TEE there is no potential compromise of the airway or need for general anesthesia for long interventions with ICE. Currently ICE can be quite useful for performing transseptal puncture, which is often needed for mitral interventions such as balloon valvuloplasty and MitraClip therapy. On the other hand, the 2D format of ICE images may make guiding transseptal puncture to a certain location on the septum difficult. Figure 16-7 shows how ICE complements fluoroscopy for standard transseptal puncture. FIGURE 16-6 Visualization of all valves on intracardiac echocardiography (ICE). FIGURE 16-7 Imaging guidance of transseptal puncture with intracardiac echocardiography (ICE). Echocardiography is widely recognized as the preferred imaging tool for the evaluation and management of patients with stenotic, 40 regurgitant, 41 and prosthetic valves. 42 The development of real-time 3D echocardiography has resulted in improved spatial resolution on images and enhanced visualization of the morphologic features of the cardiac valves.43,44 Because of its universal availability, portability, and capacity to provide high-quality, reliable physiologic and morphologic information, echocardiography has been embraced with enthusiasm in the catheterization laboratory to provide guidance for transcatheter interventions for structural heart disease.45–50 In addition to helping with patient selection, procedure guidance, and prevention and recognition of procedure complications, echocardiography can enable a clearer understanding of the hemodynamic and physiologic consequences of the devices being deployed. 51 Both transthoracic and transesophageal echocardiography play a role in the management of patients undergoing transcatheter valve interventions, and all modalities of ultrasound, including 2D, 3D, and Doppler techniques, are being used in interventional suites and in the hybrid operating rooms dealing with valvular heart disease transcatheter interventions. Figure 16-8 illustrates the use of 3D TEE for TAVI guidance. FIGURE 16-8 Real-time three-dimensional transesophageal echocardiography guidance for transcatheter aortic valve implantation. Because of the aging of the population, with the consequent greater complexity of and higher surgical risk of potential candidates, surgical valve replacement and valve repair, traditionally the only viable options to treat advanced valvular heart disease, are being supplemented by newly developed transcatheter valve procedures52–54 (see Tables 16-1 and 16-2). Percutaneous balloon aortic valvuloplasty was first described by Cribier et al 55 in 1986. The procedure was carried out in three elderly patients with acquired severe aortic stenosis. Transvalvular systolic pressure gradient was considerably decreased at the end of the procedures, during which there were no complications. Cardiac imaging, in the form angiography and echocardiography, confirmed increased valve opening. The initial enthusiasm for this technique56–62 was tempered by the recognition that the hemodynamic and clinical improvement was short lived. During follow-up, Doppler echocardiographic results demonstrated a trend toward the preprocedural severity of the aortic stenosis. Progression of restenosis assessed by Doppler echocardiography was accelerated in the patients who subsequently died or underwent repeat balloon valvuloplasty or aortic valve replacement (AVR). 63 Restenosis after balloon aortic valvuloplasty is common, occurring in as many as 50% or more of patients during the first year; histologic changes in restenosed valves differ from those seen in calcific aortic stenosis, with granulation tissue, fibrosis, and ossification being present. 64 An encouraging factor was the demonstration that AVR could be performed with a low mortality rate, excellent palliation of symptoms, and prolongation of survival in selected high-risk patients with a history of previous balloon aortic valvuloplasty. 65 One center has reported that repeat balloon valvuloplasty is a viable treatment strategy in patients who have severe calcific aortic stenosis and are not candidates for surgery, because it provides a median survival rate of approximately 3 years and maintains clinical improvement, 66 although this is not the experience at most centers. Aortic balloon valvuloplasty has enjoyed a revival thanks to the interest in and development of TAVI. As discussed later, balloon valvuloplasty is used during TAVI to facilitate the passage of the crimped aortic valve through the narrow aortic valve orifice. Aortic balloon valvuloplasty can also be used to improve hemodynamics in some patients who are not surgical candidates and have severe AS, and a proportion of these patients improve to a point at which AVR can be performed. Use of balloon valvuloplasty as a bridge to TAVI will provide further options to high-risk patients who cannot be “bridged” to conventional AVR.67–70 The key uses of imaging for aortic balloon valvuloplasty are summarized on Table 16-5. In 1984 Inoue et al 71 reported a new balloon catheter technique that allows mitral commissurotomy without thoracotomy. The procedure was successful in five of the six patients with mitral stenosis so treated. Two-dimensional echocardiography showed a marked to moderate degree of dilation of the mitral orifice in each patient. Balloon mitral valvotomy (BMV) was rapidly accepted, refined, and expanded as an effective nonsurgical procedure to treat patients with mitral stenosis, including those with pliable valves, those with previous commissurotomy, and even those with mitral calcification. 72 In patients with rheumatic mitral stenosis, transcatheter mitral valve balloon valvotomy has virtually replaced surgery as the preferred method to improve mitral valve area.73–77 The central role of echocardiography in patient selection for BMV was initially described by Wilkins et al. 78 The appearance of the mitral valve on the predilation echocardiogram was scored for leaflet mobility, leaflet thickening, subvalvular thickening, and calcification. A high score, indicating advanced leaflet deformity, on predilation imaging had a suboptimal outcome, whereas a low score (a mobile valve with limited thickening) was associated with an optimal outcome. In addition, the presence of commissural calcium has been found to be a strong predictor of outcome after percutaneous mitral balloon valvotomy. Patients with evidence of calcium in a commissure have a lower survival rate and a higher rate of later mitral valve replacement. 79 BMV can be performed with fluoroscopic guidance alone with the self-centering Inoue balloon catheter ( Figure 16-9); however, even an experienced operator can be misled by radiographic tissue landmarks. The addition of real-time TEE during BMV facilitates the success and safety of this procedure by guiding the transseptal puncture and assisting with navigation of the dilating balloon catheters across the stenotic mitral valve. 80 In addition to guiding the manipulation of catheters, real-time TEE is useful for confirming the efficacy of valvotomy and for detecting and managing complications. Severe mitral regurgitation is a relatively infrequent complication of Inoue balloon valvotomy; it results from disruption of valve integrity ( Figure 16-10), including chordal rupture and leaflet tearing. 81 TEE facilitates careful balloon positioning so as to avoid chordal rupture. The additional value of 3D echocardiography in patients with mitral valve stenosis undergoing balloon valvuloplasty is now well recognized.82,83 The 3D TEE method enables a better description of the mitral valvular anatomy, especially after BMV. 84 FIGURE 16-9 Fluoroscopic Guidance of balloon mitral valvotomy.
Imaging Guidance of Transcatheter Valve Procedures
Valvular Heart Disease in the Era of Transcatheter Valve Procedures
Approaches to Imaging Guidance
Fluoroscopy and Angiography
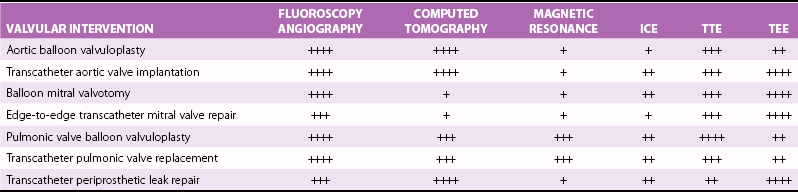
Both fluoroscopy and angiography are extensively used during this procedure. The gantry position is critically important to provide a view for valve deployment. A shows such a view, in which the undeployed stent-valve is perpendicular to a line drawn at the base of all three aortic sinuses. B, Aortography to document that the undeployed valve is approximately 50/50 below and above the valve plane. C, Balloon inflation with stent expansion and deployment of a SAPIEN valve (Edwards Lifesciences Corporation, Irvine, California). D, Immediately after deployment, a well-expanded stent-valve with the calcified aortic cusps pushed to the side can be seen.
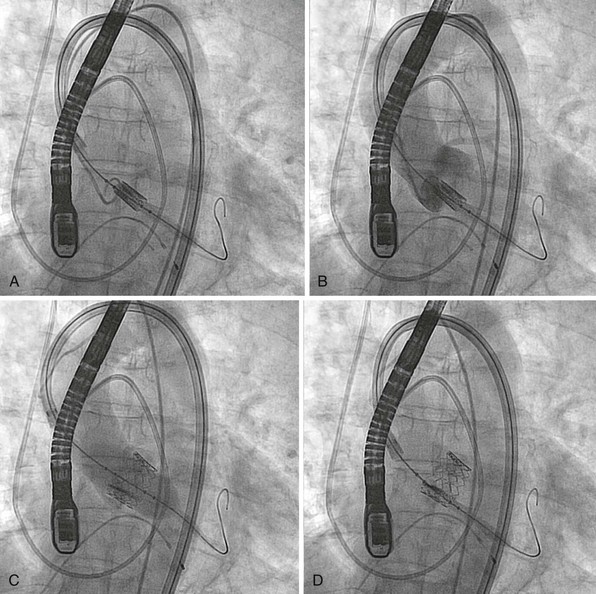
Innovations in image guidance of valvular heart disease interventions are developing new technologies that are integrated in the procedure room. A, A three-dimensional (3D) reconstruction from a rotational angiographic acquisition with the flat x-ray detector making a 180-degree arc around the patient. Like a computed tomography scan, it can be segmented to show chambers and valves and can be used for fluoroscopic registration with image overlay. B, The combination of 3D transesophageal echocardiographic imaging with fluoroscopy is being used here to guide a MitraClip implantation (Abbott Laboratories, Abbott Park, Illinois). The radiographic and 3D ultrasound images are registered to provide a similar perspective.
Multidetector Computed Tomography
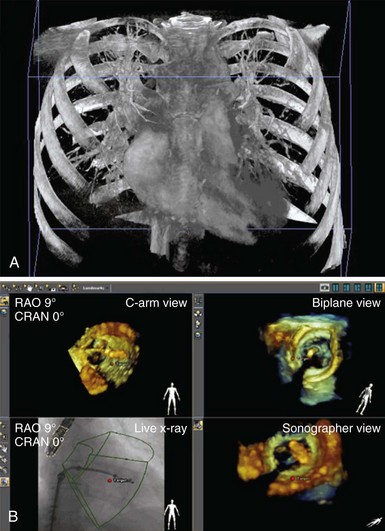
CT images illustrating short- and long-axis views and three-dimensional (3D) volumetric reconstructions before (A to C)and after (D to F) transcatheter aortic valve implantation. In short-axis views, A illustrates the traced aortic valve area, and D the deployed valve (arrow). In long-axis views, B shows the aortic annular dimension, and E the deployed prosthesis in relation to the left main coronary artery (arrow). The 3D volumetric reconstructions panel C illustrate the stenotic aortic valve seen in short axis (C) and the stent of the deployed valve (F). AO, Aorta; LA, left atrium; LVOT, left ventricular outflow tract. (Images courtesy Dr. Robert Quaife, University of Colorado Denver.)
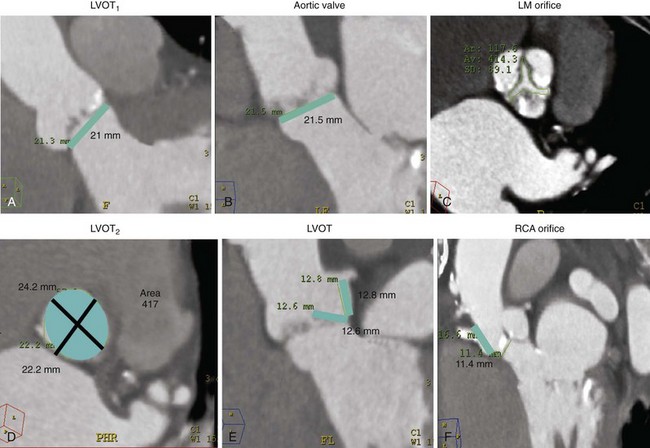
A and B illustrate the two orthogonal measurements of the aortic annulus that are used for prosthesis sizing (LVOT1 and LVOT2). In A, the blue bar corresponds to a annular dimension of 21 mm; in B, the annular dimension is 21.5 mm. C, The aortic valve area measured in short axis. D, The area at the level of the aortic annulus is depicted as a blue ellipse. The maximal and minimal orthogonal diameters at this level are also noted (black lines). E, Blue bars represent the distance from the aortic valve plane to the orifice of the left main trunk, 12.8 mm, and the left coronary leaflet length, 12.6 mm. F, The distance from the valve to the right coronary ostium (RCA) is measured at 11.4 mm (blue bar). (Images courtesy Dr Robert Quaife, University of Colorado Denver.)
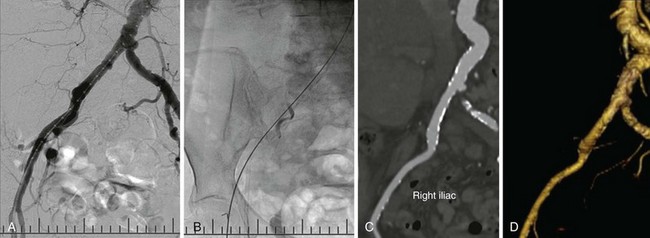
This procedure involves large delivery catheters and requires assessment of potential vascular access routes. Different modalities are used, including digital angiography (A), fluoroscopy with rigid guide wire to assess whether straightening of the vessel is needed (B), CT angiography with multiplanar reformatting to allow coaxial assessment of vessel diameter (C), and CT angiography three-dimensional reconstruction to assess tortuosity (D).
Cardiac Magnetic Resonance
Intracardiac Echocardiography
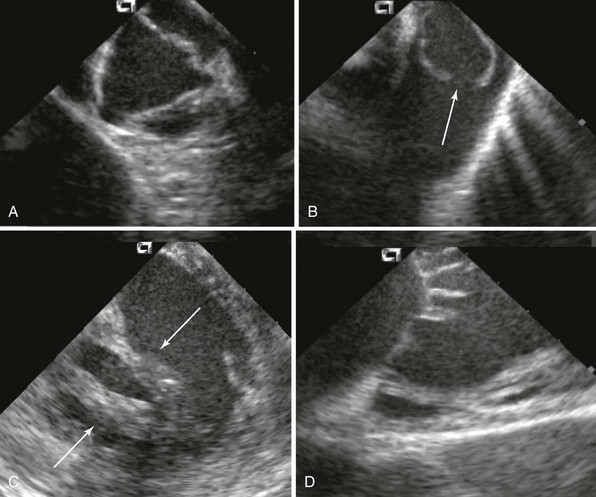
ICE provides detailed visual assessment of all valves, including valve leaflets and subvalvular apparatus. A, Normal aortic valve. B, Congenital pulmonic valve stenosis with doming (arrow). C, Rheumatic mitral stenosis with extensive chordae thickening and shortening (arrows). D, Normal tricuspid valve. Catheter positions in A through C were various locations within the right ventricle; in D, catheter was in the right atrium.
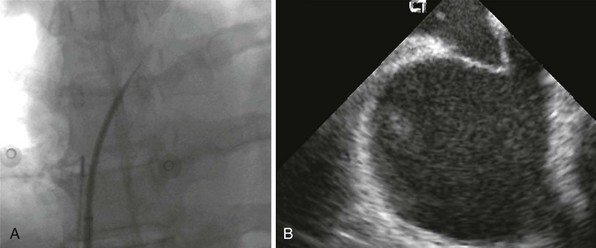
The safety and precision of transseptal catheterization have been greatly improved by the use of ultrasound, including ICE. A shows a fluoroscopic image of the transseptal needle extending past the tip of the dilator of the transseptal sheath. B shows the corresponding ICE image that reveals the tenting of the septum but no crossing of the needle into the left atrium. With this knowledge from imaging, the operator can safely push with more force to complete the puncture.
Transthoracic and Transesophageal Echocardiography
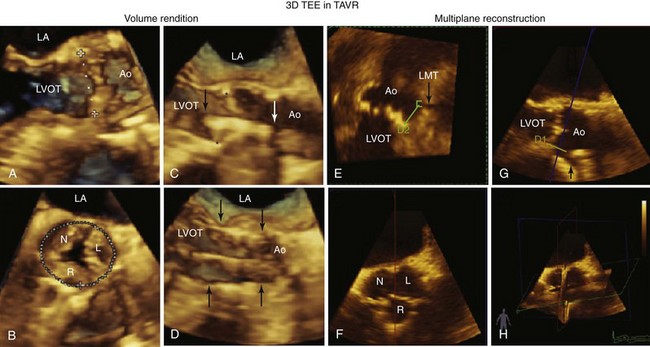
A to D, The use of volume rendition mode three-dimensional (3D) transesophageal echocardiography guidance of the implantation procedure. A, The dotted line represents a 3D “measurement on glass” of the aortic annulus. B, The measurement on glass of the circumference of the aortic annulus C, The crimped prosthesis (arrows) is being centered to the aortic annulus (asterisks). D, The deployed prosthesis is delineated by the arrows. E to H, The multiplane reconstruction mode being used in this case to delineate the distance from the left main trunk (LMT, black arrow) to the aortic leaflets. E, The green line marks the distance (D2) from the base of the left coronary cusp to the LMT. In G, the line (D1) marks the same distance in an orthogonal plane. F illustrates the 3D short-axis view of the aortic valve depicting the right (R), left (L), and noncoronary (N) cusps. H shows the three orthogonal planes used to obtain these views. Ao, Aorta; LA, left atrium; LVOT, left ventricular outflow tract.
Transcatheter Treatment of Valve Disease
Transcatheter Balloon Valve Procedures
Balloon Aortic Valvuloplasty
Balloon Mitral Valvotomy
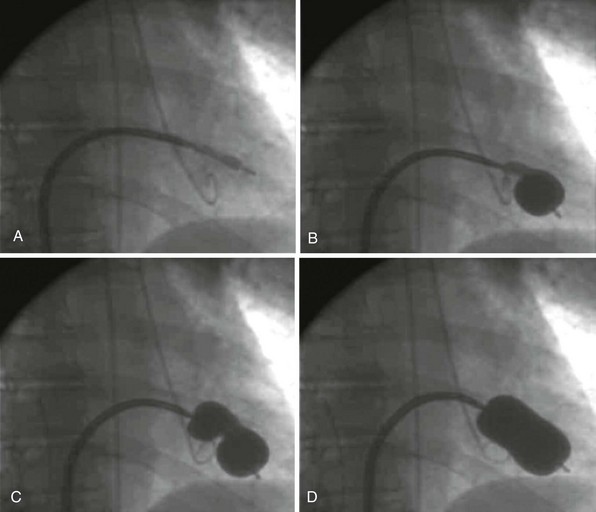
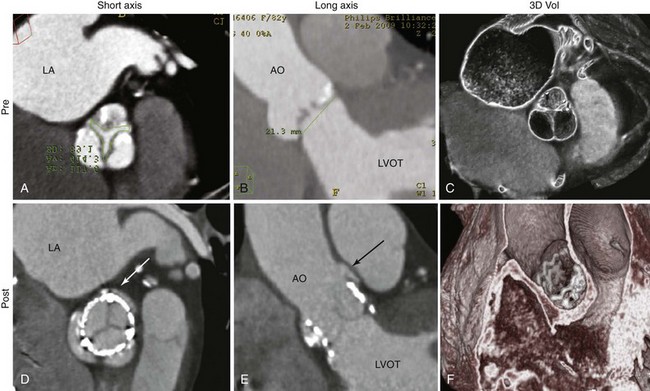
Fluoroscopy can be the main imaging modality to guide the performance of the procedure using the Inoue balloon catheter. A, Crossing of the catheter from the left atrium into the left ventricle is often straightforward. B, Inflation of the balloon first occurs in the distal balloon, allowing the catheter to be pulled back to engage the valve orifice. C, Further inflation enlarges the proximal part of the balloon, which “locks” the mitral valve orifice, and the stenotic orifice accentuates a “dog-balloon” appearance. D, The final full inflation straightens the midportion of the balloon, providing the force to split the fused commissures.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Imaging Guidance of Transcatheter Valve Procedures
Only gold members can continue reading. Log In or Register to continue