Fig. 19.1
(a) Active extravasation (arrow) of contrast seen arising from a branch of the left internal iliac artery in a patient with multiple pelvic fractures after blunt trauma. (b) Successful coil embolization (arrow) of the bleeding vessel. Initial diagnosis of active bleeding was made with CTA
Large arterial wall defects may be amenable to endovascular stent-grafting with covered stent-grafts in order to exclude the injured segment. Arterial access may be established through the ipsilateral or contralateral femoral system, as well as through an axillary approach. Delivery of stent-grafts typically requires relatively large arterial sheaths and may be made more difficult by calcification of the potential access sites. In addition, adequate landing zones are required proximally and distally to the area of injury in order to ensure correct stent placement and to avoid stent-graft migration.
Failure of endovascular attempts to control ongoing arterial hemorrhage requires surgical exploration. Temporary aortic occlusion balloons placed in the angiography suite may be used under such circumstances to slow the rate of hemorrhage while operative resources are mobilized [10].
The role for angiography in penetrating iliac arterial injury is limited. The majority of these patients present in hemorrhagic shock and with multiple other associated injuries which require immediate operative intervention [2, 10, 12, 13, 23, 24, 26, 27, 34, 53]. While great advances in endovascular therapy have taken place over the past 20 years, most trauma centers still lack hybrid interventional-operative suites to attempt combined open and endovascular treatment [10].
Although fortunately a rare complication, iatrogenic injury to the iliac arteries most commonly occurs either as the result of laparoscopic trocar insertion or pedicle screw placement during lumbar spine surgery. Successful placement of a covered, self-expanding, or balloon-expandable endovascular stent-graft to exclude the site of injury has been reported [14, 54]. Unlike patients who suffer iliac artery injuries in the field, these injuries occur in the operating suite and resuscitation can begin immediately, and the incidence of concomitant injury and physiologic derangement is low. Emergent transport to the angiography suite for endovascular treatment may be preferred over open management of these injuries in this particular patient population.
19.6.2 Operative Treatments
Patients with penetrating injury require immediate operative management. These patients, along with patients who have a blunt mechanism and indication for operation, should be brought to the operating room with adequate stores of blood and blood products immediately available. Normothermic measures should be continued throughout the operative period. The patient is placed in the supine position and is prepped and draped in the standard trauma fashion—from chin to knees and to the OR table on the sides. Both axillae should be within the field if the need for axillofemoral bypass arises. A groin towel is placed with care not to obscure access to the femoral arterial system on either side of the patient. Preoperative antibiotics should be administered prior to incision.
Exposure begins through a midline laparotomy incision extending from the xyphoid to the symphysis pubis. Once the peritoneal cavity has been entered, packing of solid organ injuries should proceed along standard trauma principles. Presence of a zone III retroperitoneal hematoma in the setting of penetrating trauma is not uncommon in patients with iliac arterial injury and requires exploration. If the iliac artery bleeds freely into the abdomen, direct manual compression to the artery lesion or proximal artery can stop bleeding until proximal and distal control can be achieved.
Exposure of the iliac system is best achieved through a left-to-right medial visceral rotation (Mattox maneuver) [55]. The peritoneal attachments of the left colon are mobilized along the avascular line of Toldt, and the left colon is rotated medially to expose the aorta and iliac vessels (Fig. 19.2). Alternatively, if injury to the right iliac artery is suspected, right-to left medial visceral rotation provides easy access to the right common and external iliac arteries. Care must be taken to avoid injury to the ureters during dissection.
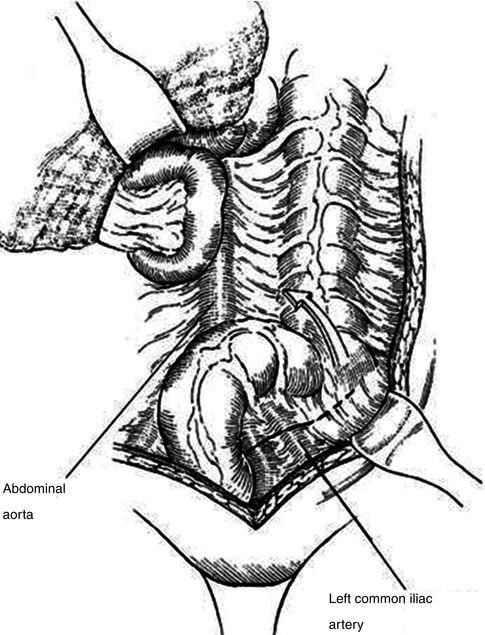

Fig. 19.2
Left-to-right medial visceral rotation (Mattox maneuver). Mobilization of the left colon along the line of Toldt provides excellent access to the abdominal aorta (Adapted from Lee and Bongard [4], with permission)
The discovery of massive hemorrhage on entering the abdomen requires immediate action to attempt to gain proximal and distal control of the bleeding vessels. Temporary manual compression of the distal aorta against the vertebral bodies and of the common femoral arteries just beyond the inguinal ligament may slow the rate of hemorrhage while formal proximal and distal control can be established [4, 24]. This is best achieved through pelvic vascular isolation either with vascular clamps or vessel loops.
Proximal control is obtained by carefully placing vascular clamps across the distal aorta and inferior vena cava proximal to their bifurcations. Distal control is obtained by placing a single clamp across the distal external iliac artery and vein bilaterally [4, 56, 57]. This maneuver allows for further identification of areas of both arterial and venous injury. As injuries are identified, the clamps are moved more near to the area of injury thus reestablishing flow to uninjured vessels.
Injury to the distal external iliac artery may require an inguinal incision to gain adequate distal control. This incision should be made longitudinally and can be extended distally to transect the inguinal ligament if necessary. Alternative proximal control can be obtained by transecting the hepatoduodenal ligament and cross-clamping the supraceliac aorta. This not only compromises mesenteric and renal blood flow but also allows for greater collateral flow to the injured artery and should only be used as a temporizing measure while more proximal control can be obtained closer to the site of injury.
Once the area of injury has been identified and isolated, devitalized tissue should be debrided to allow for a full assessment of the extent of injury and allow for proper choice of the type of repair that should be attempted. Simple disruptions involving less than 50 % of the circumference of the arterial wall may be closed primarily in a transverse fashion with interrupted polypropylene suture. Attempting primary repair of more extensive lesions may result in stenosis at the site of anastomosis.
Such lesions may be treated with excision of the injured segment and end-to-end anastomosis. This should only be attempted if mobilization of the proximal and distal artery can provide for a tension-free repair. Distances greater than 1 cm are generally not amenable to end-to-end anastomosis [4]. Autologous saphenous vein grafts are rarely large enough caliber for use in the iliac arteries [10]. Alternatively, autologous femoral vein may be used for interposition grafting of the iliac arteries [58, 59]. The femoral vein is typically of adequate diameter for interposition grafting of the iliac arteries. Unlike the saphenous vein, its length may be of concern should a particularly long segment of artery require excision. Either the ipsilateral or the contralateral superficial femoral vein is chosen if concomitant injury is absent. Also, consider the likelihood of adequate venous outflow from the chosen donor leg if distal injuries are present. The proximal and distal superficial femoral vein is clamped and side branches are ligated. The homograft is then excised leaving adequate cuffs near both clamps for definitive closure of the venous stumps. Valves are stripped or the direction of the conduit is reversed before reanastomosis. The interposition graft is placed using polypropylene sutures with care to avoid excess tension, torque, or redundancy.
If a tension-free repair cannot be accomplished with the available length of femoral autograft, the use of polytetrafluoroethylene (PTFE) grafts provides an excellent option for reconstruction. These are typically readily available in most hospitals in various sizes. As compared with grafts for more distal arteries, the use of PTFE grafts in the iliac system has better primary and secondary patency rates [60]. An appropriately sized PTFE graft is anastomosed proximally and distally with 4-0 or 5-0 polypropylene suture. The clamps are flashed both proximally and distally to minimize thromboembolism prior to completing the anastomosis. Reconstruction of more complex injuries occurring near areas of bifurcation can be accomplished either with arterial transposition or extra-anatomic bypass.
Arterial transposition involves removing the common iliac artery near the aorta, excising the injured segment, and reimplanting the arterial stump to the contralateral common iliac artery. This technique allows for tension-free repair of injuries occurring near the aortic bifurcation. To accomplish this, the aortic cross-clamp is carefully placed immediately proximal to the aortic bifurcation. Vascular clamps are then placed across both common iliac arteries distal to the area of injury and with enough space on the contralateral side to allow for easy reanastomosis. The injured vessel is divided near the aorta and the proximal iliac artery stump is closed with 3-0 or 4-0 polypropylene suture in two layers (Fig. 19.3). The injured portions of the explanted artery are debrided, and an arteriotomy is made in the contralateral common iliac artery. The anastomosis is carried out with 4-0 or 5-0 polypropylene suture [4].
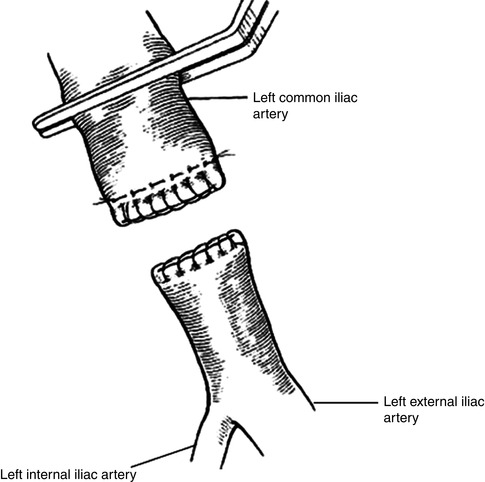

Fig. 19.3
Two-layer closure of iliac arterial stump. Polypropylene suture is used to close the artery in a horizontal mattress stitch. A second running layer is imbricated over the distal arterial stump (Adapted from Lee and Bongard [4], with permission)
Injuries to the external iliac artery near the iliac bifurcation can be repaired using the uninjured ipsilateral hypogastric artery as an interposition graft. Proximal control is obtained with a vascular clamp placed carefully across the proximal common iliac artery. Care must be taken during this dissection to identify and protect the ureter as it crosses into the pelvis at the iliac bifurcation. Control of outflow may require a separate inguinal incision and division of the inguinal ligament should mobilization transabdominally fail to provide adequate length to perform a tension-free repair. The ipsilateral hypogastric artery is then mobilized and divided at the level of the middle hemorrhoidal artery [4]. The injured segment of the external iliac artery is then excised and both proximal and distal stumps are oversewn in two layers with polypropylene suture. The hypogastric artery is then reimplanted to the external iliac artery distal to the area of injury (Fig. 19.4).
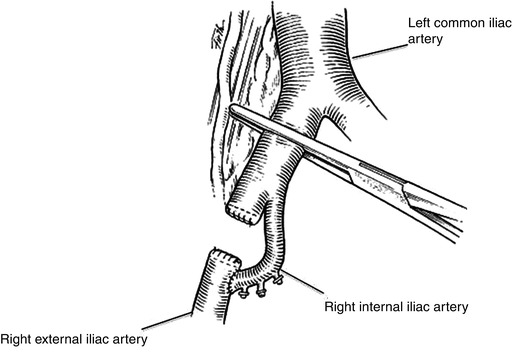

Fig. 19.4
Ipsilateral hypogastric arterial transposition is used to perfuse the lower extremity in patients with injuries to the proximal external iliac artery (Adapted from Lee and Bongard [4], with permission)
The advantage of such arterial transposition techniques is that they avoid the need for prosthetic material when operating in a contaminated field. The most obvious drawback to such techniques is that they require longer operative times and are more technically complex than either simple repair or prosthetic interposition grafting.
Concomitant injury to visceral or urologic structures is common [4, 12, 23, 26, 27]. The use of prosthetic material for vascular repair in the setting of abdominal contamination remains controversial. In the largest series to date, Burch reported the use of prosthetic arterial interposition grafts in five patients with associated colorectal injuries. Of these patients, there were no graft infections and all grafts remained patent without thrombosis [10, 23]. In the setting of visceral injury, thorough irrigation of the peritoneal cavity should precede any definitive vascular repair. The avoidance of prosthetic material in the setting of gross fecal contamination is reasonable as long as this decision does not significantly lengthen the operative time or exceed the technical expertise of the surgeon.
Extra-anatomic revascularization with either femoral-femoral or axillofemoral bypass represents an additional option in the setting of gross contamination. These techniques have been long used in the non-trauma population in the setting of contamination; however, they are generally time-consuming and have lower long-term patency rates than local repairs [23]. In the majority of patients with iliac artery injuries, extra-anatomic bypass should not be attempted at the initial operation and should be reserved for only those with significant fecal contamination that makes the use of local prosthetic grafts suboptimal.
Attempts at definitive repair of iliac artery injuries at initial operation must be made judiciously. Asensio and colleagues have demonstrated a reliable set of variables, including Injury Severity Score (ISS) ≥25, systolic blood pressure <70 mmHg, pH <7.10, or temperature <34 °C, the presence of any which predict poor outcomes so a damage control approach is recommended [37]. Given the significant physiologic derangement often seen at initial presentation, the use of damage control techniques, including temporary intravascular shunts (TIVS), should be utilized if patients demonstrate significant acidosis or hypothermia [2, 23, 26, 61].
The TIVS provides fast and reliable perfusion to the lower extremity in situations where ligation would have previously been considered for uncontrolled hemorrhage in the unstable patient. The use of these synthetic shunts both controls hemorrhage and prevents critical limb ischemia. Whereas unilateral internal iliac artery ligation is generally well tolerated, ligation of the external iliac artery as a lifesaving maneuver results in limb loss in 50 % of patients, and there is an associated 90 % overall mortality [6, 23, 62]. TIVS can range in complexity from small chest tubes or red-rubber catheters to heparin-bonded Argyle shunts designed for such a purpose. Selection of a device for TIVS should be based upon their rapid availability and arterial caliber. Shunts are placed after first obtaining proximal and distal control of the injured segment. Thrombectomy is performed with appropriately sized Fogarty balloons and the shunt is placed in the proximal artery. The shunt is then flushed with blood and placed in the distal artery. Both ends are secured using heavy silk ties [62]. Arterial clamps are then removed and blood flow to the distal extremity assessed by confirming distal Doppler signals. Definitive repair is generally attempted 24–48 h after initial operation once the patient has been adequately warmed and resuscitated. Fasciotomies should be performed in the distal leg anytime prolonged ischemia to the extremity is suspected. The specific order in which these multidisciplinary repairs should proceed is discussed elsewhere in this textbook.
19.7 Postoperative Management
Patients with iliac arterial injuries or who have undergone iliac artery repair should be monitored initially in an intensive care setting. Peripheral pulses on the side of injury should be carefully monitored and documented. Suspicion of a developing compartment syndrome should prompt a return to surgery for fasciotomy. Particular attention should be paid to the fluid status of these patients as most have suffered massive blood loss with varying degrees of resuscitation during treatment. Use of central venous catheters to monitor central venous pressure and guide ongoing fluid replacement is commonly indicated.
Routine systemic anticoagulation for patients who have undergone large-vessel (aorta, iliac) reconstruction is not indicated. For those patients who have undergone more distal bypasses, the use of systemic anticoagulants should be weighed against the risk of bleeding depending on what other injuries the patient has suffered.
For patients without hollow viscous or urologic injury, standard perioperative antibiotic therapy alone is warranted. Broad-spectrum antibiotic therapy is recommended for those with intestinal spillage or an open abdomen. For patients with gastrointestinal contamination and synthetic material used for reconstruction, prophylactic antibiotic therapy should be continued longer.
19.8 Complications and Pitfalls
The vast majority of mortality associated with iliac artery injuries results from complications of hemorrhagic shock within the first 24 h of injury [2, 23, 25, 26, 61, 63]. Failure to control hemorrhage or to adequately resuscitate these frequently moribund patients leads to poor outcomes.
Vascular complications related to the repair of iliac artery injuries are dependent upon the type of repair. In the largest series to date, Burch et al. reported an overall arterial complication rate of 10.5 % among patients surviving greater than 24 h. In this series, a single patient undergoing end-to-end anastomosis experienced arterial thrombosis postoperatively. Four patients (25 %) treated with PTFE interposition grafts experienced graft thrombosis; however, all grafts were successfully opened at a second operation. Four patients (67 %) treated with ligation of the common or external iliac artery with extra-anatomic bypass suffered arterial complications, including graft thrombosis (33 %), compartment syndrome (50 %), and ultimate amputation (50 %) [23]. It should be noted that this later group of patients had suffered significantly greater blood loss and were much more likely to remain hypotensive following repair than in other treatment groups.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


