Hybrid Thoracoabdominal Aortic Aneurysm Repair
JEFF MATHEW, PRAVEEN C. BALRAJ, and LOAY S. KABBANI
Presentation
A 65-year-old female, who is a 50-pack-year smoker, is referred by her primary care physician who felt a pulsatile abdominal mass. The patient is not experiencing pain and has no complaints other than some back discomfort. A review of the patient’s past medical history reveals poorly controlled hypertension and chronic obstructive pulmonary disease (COPD). The patient is partially dependent on 2 L/min of oxygen at home. She can walk up one flight of stairs, but gets dyspneic. She did not have any previous abdominal surgeries. Physical examination reveals a nontender abdomen, with a pulsatile epigastric mass left of midline.
Differential Diagnosis
The most common etiology of a pulsatile epigastric mass is aortic aneurysm. Much less common is superior mesenteric aneurysm. Other entities that transmit the aortic pulse to the abdominal wall and can mimic abdominal aortic aneurysms are tumors, pancreatic pseudocysts, hepatomegaly secondary to right heart failure, and an enlarged spleen. Keep in mind, though, that in a thin patient, a normal aorta can be palpated and mistaken for an abnormal pulsating mass. Conversely, the aneurysm cannot be palpated in 24% patients with a greater than 5 cm abdominal aneurysm.
Workup
The patient’s serum creatinine is 0.9 mg/dL. A cardiac stress test was performed 6 months prior to admission for atypical chest pain and revealed no significant ischemia. Pulmonary function tests report an FEV1 of 1.2 L/min (30% predicted), an FVC of 1.7 L (35% predicted), an FEV1/FVC of less than 0.7, and a 20% improvement after using bronchodilators.
CT Scan
A thin-slice (1.5 mm) CT angiogram (CTA) is ordered. A 7.0-cm nonruptured thoracoabdominal aneurysm (TAAA) is seen, extending from the midthoracic aorta to the level of the renal arteries (type V) (Fig. 1). There is a moderate amount of intraluminal thrombus in the aneurysm sac. The ostium of the superior mesenteric artery (SMA) has moderate amount of atherosclerotic disease, but no stenosis. The celiac artery has a high-grade stenosis at its origin, and the inferior mesenteric artery (IMA) is occluded. Both renal arteries are patent. The femoral and iliac arteries are of good caliber.
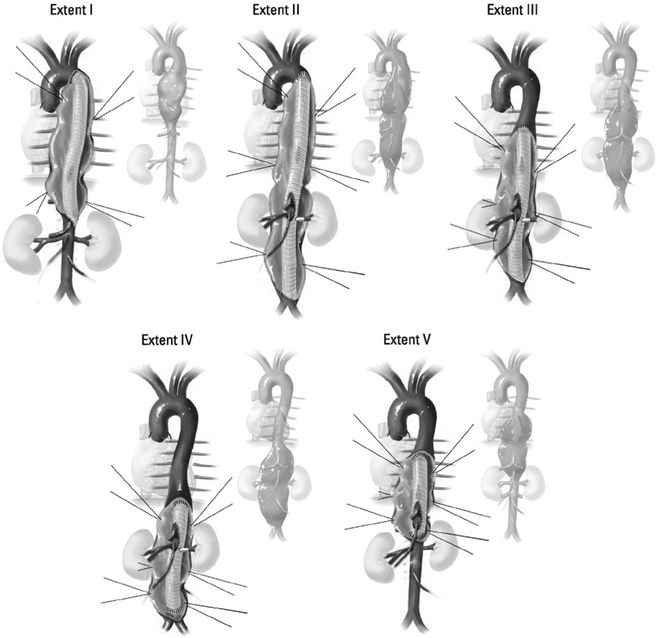
FIGURE 1 TAAAs, classified by the proximal and distal extent of the aortic aneurysm.
(Adapted from Frederick JR, Woo YJ. Thoracoabdominal aortic aneurysm. Ann Cardiothorac Surg. 2012;1(3): 277–285.) Type I involves most of the descending thoracic aorta from the origin of the left subclavian to the suprarenal abdominal aorta. Type II is the most extensive, extending from the subclavian to the aortoiliac bifurcation. Type III involves the distal thoracic aorta to the aortoiliac bifurcation. Type IV is limited to the abdominal aorta below the diaphragm. Type V extends from the distal thoracic aorta including the celiac and superior mesenteric origins, but not the renal arteries.
Discussion
Unfortunately, very few patients with aortic aneurysms present with symptoms prior to an acute aortic event, with most events occurring in the absence of heralding symptoms. Symptoms may include a dull abdominal or thoracic pain or pressure, or, in the event of rupture, patients may experience an acute tearing or stabbing chest or back pain. As with other forms of aortic aneurysms, TAAAs are usually detected during an examination for other conditions. When working up the patient, it is important to perform a comprehensive history and physical examination and investigative studies. Of particular importance is assessment of cardiac, respiratory, and renal function (Fig. 2).
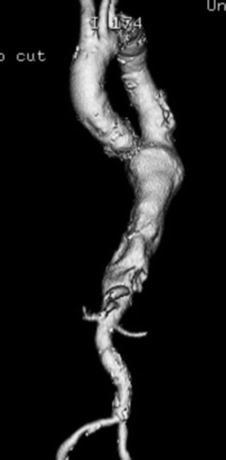
FIGURE 2 CTA showing TAAA.
CTA is an expedient and extremely accurate way to diagnose aortic aneurysms. When performing imaging for a suspected aortic disease, it is important to delineate the whole thoracic and abdominal aorta and to rule out other areas of aneurysmal dilation (found in up to 12% of patients). Renal function must be evaluated prior to imaging with intravenous contrast. CTA provides most of the diagnostic information needed; it can reveal the presence of hostile angles, atherosclerosis of branch vessels, and the presence of thrombus in the aorta. It can also provide measurements for endovascular repair planning, in terms of landing zones, location of branch vessels, size of endografts needed, and whether access vessels are of sufficient size to permit endograft deployment. CTA can also provide valuable information for open repair, such as optimal location for aortic clamping, and location/patency of branch vessels. Such knowledge gained preoperatively can transform a “hostile terrain” into a more manageable one.
Size of the aneurysm and rate of growth are important predictors of rupture. Size criteria for repair of TAAA are not as clearly defined as for infrarenal AAA. Adjustment for body surface area needs to be incorporated into the decision making when it comes to the optimal time for repair. Most surgeons would consider repairing a TAAA in an average-sized patient when it reaches 6 to 6.5 cm in diameter, with a lower threshold for aneurysms in patients with connective tissue disorders. According to Elefteriades, a descending thoracic aorta that has reached 6 cm maximal diameter faces the following yearly rates of devastating adverse events: rupture (3.6%), dissection (3.7%), and death (10.8%). A 7-cm aneurysm has a lifetime rupture risk of 43%. Overt symptoms and yearly aneurysm growth (greater than 10 mm/year) are other indications for repair. Aortic rupture is a catastrophic event with extremely high morbidity and mortality.
The treatment of TAAA is contingent upon the balance between the risk of rupture and the risk associated with operative repair. Treating TAAA is more complicated than is managing infrarenal aortic aneurysms, because the mesenteric and renal vessels are contained within the repair zone. There are three operative approaches to consider: open, endovascular, and hybrid repair.
Open surgical repair is the traditional modality and the gold standard for managing this aneurysm. However, the thoracoabdominal incision carries significant morbidity and is associated with a high mortality rate in a patient who has significant pulmonary insufficiency.
Use of fenestrated endografts provides an alternative option. It avoids a large, traumatic incision and may be better tolerated in high-risk patients. This approach, however, is still in its infancy. Fenestrated endografts are offered in only a few select centers in the United States, though more centers are beginning to utilize this procedure. Our patient has significant mural thrombus in the vicinity of her SMA, her celiac artery is severely diseased, and her IMA is not patent. There is a serious risk of embolizing thrombus into the SMA while attempting to cannulate the SMA and placing the covered stent, making an endograft a less attractive alternative (Fig. 3).
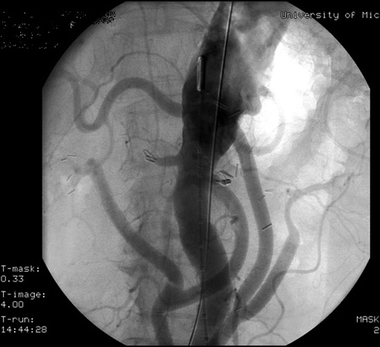
FIGURE 3 Angiogram following four-vessel debranching documenting patency of bypasses.



