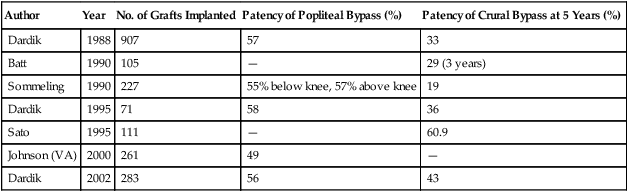
Dardik, in his initial experience with 907 bypasses performed for critical limb ischemia in 95% of patients, found a 5-year assisted primary patency rate of 57% for femoropopliteal and 33% for femorocrural bypasses. After the graft was modified, the same group reported on 71 additional infrageniculate bypasses, with a 58% primary patency for below-knee popliteal reconstructions and 36% for crural reconstructions. Secondary patency was 64% and 41%, respectively. Results of patency and limb salvage from other studies of HUV were similar for bypasses above the knee but were variable for more distal bypasses (Table 1). TABLE 1 Patency for Human Umbilical Vein Grafts
Human Umbilical Vein and Other Biografts for Atherosclerotic Lower Extremity Occlusive Disease
Human Umbilical Vein Grafts
Patency Data
Author
Year
No. of Grafts Implanted
Patency of Popliteal Bypass (%)
Patency of Crural Bypass at 5 Years (%)
Dardik
1988
907
57
33
Batt
1990
105
—
29 (3 years)
Sommeling
1990
227
55% below knee, 57% above knee
19
Dardik
1995
71
58
36
Sato
1995
111
—
60.9
Johnson (VA)
2000
261
49
—
Dardik
2002
283
56
43
Thoracic Key
Fastest Thoracic Insight Engine



