12 High-Risk Percutaneous Coronary Interventions
Identifying the High-Risk PCI Patient
Angiographic Factors
The American College of Cardiology/American Heart Association (ACC/AHA) created a scoring system that classified lesions according to their complexity, likelihood of successful dilation, and the likelihood of an adverse event. Lesions were classified as either type A, B, or C, with C the highest risk lesions, based on lesion characteristics (Tables 12-1 and 12-2).
Table 12-1 The ACC/AHA Lesion Classification Scheme
| Type A Lesions |
| Type B Lesions |
| Type C Lesions |
ACC, American College of Cardiology; AHA, American Heart Association.
Table 12-2 Lesion Characteristics and the Increased Risk of Ischemic Complications (Based on Multivariate Analysis)
| Lesion Characteristic | Odds Ratio |
|---|---|
| Nonchronic total occlusion | 4.74 (2.69–8.38) |
| Degenerated saphenous vein graft | 4.18 (2.39–7.31) |
| Length ≥20 mm | 2.77 (1.51–5.09) |
| Irregularity | 1.88 (1.32–2.66) |
| Large filling defect | 1.41 (1.17–1.70) |
| Length 10–20 mm | 1.88 (1.26–2.82) |
| Moderate calcification with angulation >45° | 4.44 (1.24–15.96) |
| Eccentric | 2.12 (1.04–4.57) |
| Severe calcification | 2.19 (1.04–4.57) |
| Saphenous vein graft age ≥10 years | 1.81 (1.00–3.31) |
Adapted from Ellis SG, Guetta V, Miller D, et al. Relation between lesion characteristics and risk with percutaneous intervention in the stent and glycoprotein IIb/IIIa era. Circulation 1999;100:1971–1976.
In the mid-1990s, an era in which coronary stents and platelet glycoprotein IIb/IIIa inhibitors were frequently utilized, Ellis and coworkers analyzed a large database of patients undergoing PCI. Ten angiographic factors were identified that correlated with greater risk of complication. The two factors associated with the greatest increased risk were degenerated saphenous vein grafts (relative risk 4.18) and nonchronic total occlusion (relative risk 4.74). Other factors included long lesions, lesions with large filling defects, calcified angulated lesions, eccentric lesions, and old saphenous vein grafts (Table 12-2). The finding of marked increased risk in degenerated vein grafts supports the practice of using distal protection devices during PCI of such lesions.
Angiographic Risk Assessment Using the SYNTAX Score
The SYNTAX score, an angiographic grading tool to determine the complexity of coronary artery disease (CAD), was derived from pre-existing risk assessment classifications from numerous studies and expert consensus. The SYNTAX score is the sum of the points assigned to each individual lesion identified in the coronary tree with >50% diameter narrowing in vessels >1.5 mm diameter. The coronary tree is divided into 16 segments according to the AHA classification (see Fig. 12-1). Each segment is given a score of 1 or 2 based on the presence of disease, and this score is then weighted based on a chart, with values ranging from 3.5 for the proximal left anterior descending artery (LAD) to 5.0 for left main, and 0.5 for smaller branches. The branches <1.5 mm in diameter, despite having severe lesions, are not included in the SYNTAX score. The percent diameter stenosis is not a consideration in the SYNTAX score, only the presence of a stenosis from 50% to 99% diameter, <50% diameter narrowing, or total occlusion. A multiplication factor of 2 is used for non-occlusive lesions and 5 is used for occlusive lesions, reflecting the difficulty of PCI. Further characterization of the lesions adds points. (Fig. 12-1 shows the diagram of vessel segments used in the SYNTAX score.)
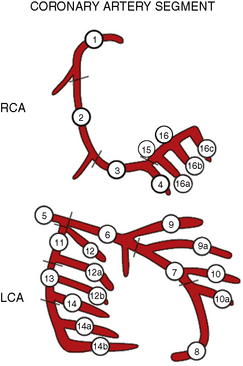
Figure 12-1 Definition of the coronary tree segments from the SYNTAX study
1. RCA (right coronary artery) proximal: from the ostium to one half the distance to the acute margin of the heart.
2. RCA mid: from the end of first segment to acute margin of heart.
3. RCA distal: from the acute margin of the heart to the origin of the posterior descending artery.
4. Posterior descending artery: running in the posterior interventricular groove.
5. Left main: from the ostium of the LCA (left coronary artery) through bifurcation into left anterior descending and left circumflex branches.
6. LAD (left anterior descending) proximal: proximal to and including first major septal branch.
7. LAD mid: LAD immediately distal to origin of first septal branch and extending to the point where LAD forms an angle (RAO [right anterior oblique] view). If this angle is not identifiable, this segment ends at one half the distance from the first septal to the apex of the heart.
8. LAD apical: terminal portion of LAD, beginning at the end of previous segment and extending to or beyond the apex.
9. First diagonal: the first diagonal originating from segment 6 or 7.
9a. First diagonal a: additional first diagonal originating from segment 6 or 7, before segment 8.
10. Second diagonal: originating from segment 8 or the transition between segment 7 and 8.
10a. Second diagonal a: additional second diagonal originating from segment 8.
11. Proximal circumflex artery: main stem of circumflex from its origin of left main and including origin of first obtuse marginal branch.
12. Intermediate/anterolateral artery: branch from trifurcating left main other than proximal LAD or LCX (left circumflex). It belongs to the circumflex territory.
12a. Obtuse marginal a: first side branch of circumflex running in general to the area of obtuse margin of the heart.
12b. Obtuse marginal b: second additional branch of circumflex running in the same direction as 12.
13. Distal circumflex artery: the stem of the circumflex distal to the origin of the most distal obtuse marginal branch, and running along the posterior left atrioventricular groove. Caliber may be small or artery absent.
14. Left posterolateral: running to the posterolateral surface of the left ventricle. May be absent or a division of obtuse marginal branch.
14a. Left posterolateral a: distal from 14 and running in the same direction.
14b. Left posterolateral b: distal from 14 and 14a and running in the same direction.
15. Posterior descending: most distal part of dominant left circumflex when present. It gives origin to septal branches. When this artery is present, segment 4 is usually absent.
16. Posterolateral branch from RCA: posterolateral branch originating from the distal coronary artery distal to the crux.
16a. Posterolateral branch from RCA: first posterolateral branch from segment 16.
16b. Posterolateral branch from RCA: second posterolateral branch from segment 16.
16c. Posterolateral branch from RCA: third posterolateral branch from segment 16.
(From Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219–227.)
The SYNTAX score algorithm then sums each of these features for a total SYNTAX score. Table 12-3 summarizes the SYNTAX grade categories. A computer algorithm (available online at www.syntaxscore.com) is then queried, and a summed value is produced. Figure 12-2 shows two patients each with three-vessel CAD but very different PCI risk based on SYNTAX scores.
Table 12-3 The SYNTAX Score Algorithm
Reprinted from Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of CAD. EuroIntervention 2005;1:219–227.
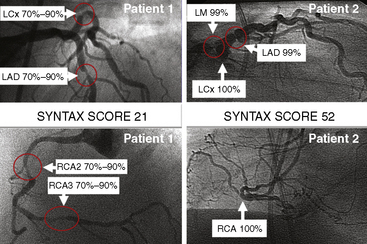
Figure 12-2 Angiographic examples of SYNTAX scores in patients undergoing PCI. Selected angiograms.
(Reprinted from Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of CAD. EuroIntervention 2005;1:219–227 with permission.)
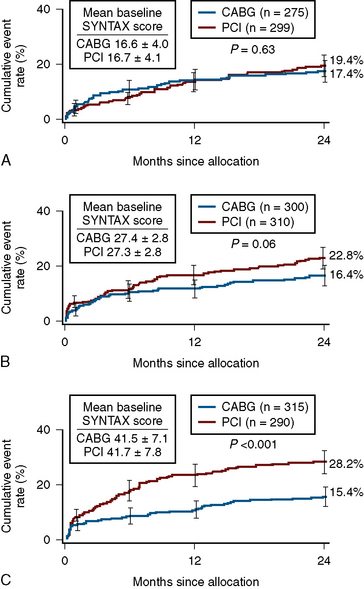
In patients with SYNTAX score <33, equal outcomes after revascularization were obtained with regard to major adverse cardiac events for both PCI and CABG. For higher SYNTAX scores, CABG had fewer adverse events than PCI. The conclusion of the SYNTAX study showed that the overall safety outcomes (death, cerebrovascular accident, MI) were similar in CABG and PCI patients at 12 months (7.7 vs. 7.6%). There was a higher rate of revascularization in the PCI group (13.7 vs. 5.9%), balanced by a higher rate of cerebrovascular accident in the CABG group (2.2 vs. 0.6%). Of note, the overall PCI major adverse cardiac and cerebrovascular event rate (MACCE) was higher (17.8 vs.12.1%) primarily due to an excess need for repeat revascularization. If one accepts a repeat revascularization as part of the natural history of PCI and not an adverse event, then the difference between revascularization strategies becomes even less (Fig. 12-3).
Patient-Related Factors
Several clinical factors can be utilized to identify high-risk PCI, such as the presence of multivessel disease, angioplasty to more than one lesion, suboptimal activated clotting time (ACT), residual stenosis above 30%, depressed ejection fraction, old age (>65 years), unstable angina and recent MI (Table 12-4). A retrospective study from the Mayo Clinic, examining the risk of PCI with the use of glycoprotein IIb/IIIa inhibitors and coronary stents, found that clinical factors such as left main or multivessel disease, an ejection fraction below 35%, or a recent MI were more important than angiographic factors for predicting complications. Other studies have identified the presence of diabetes mellitus and renal disease as indicators of high-risk patients (Table 12-5).
Table 12-4 Patient and Clinical Factors Associated With Higher Risk PCI
PCI, percutaneous coronary intervention.
Table 12-5 Elective PCI Patient and Lesion High-Risk Characteristics
CHF, congestive heart failure; SVG, saphenous vein graft.
Modified from King SB, III, Walford G, for the New York State Cardiac Advisory Committee. Percutaneous coronary interventions in New York State, 2005–2007. Albany: New York State Department of Health, April 2010;1–52; and Dehmer GJ, Blankenship J, Wharton TP, Jr., et al. The current status and future direction of percutaneous coronary intervention without on-site surgical backup: an expert consensus document from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2007;69:471–478.
PCI for AMI
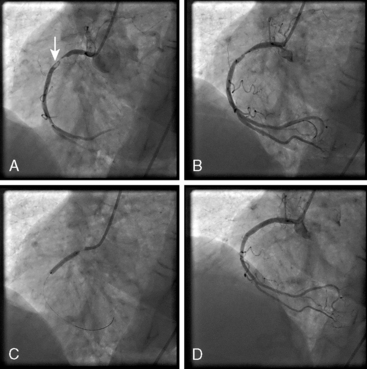
PCI for AMI can be performed before (primary PCI) or after thrombolysis (facilitated or rescue PCI). Primary PCI is the approach of choice for acute ST-segment elevation MI, but is not available in all facilities. Primary PCI for AMI is indicated in patients who present within 12 hours from the onset of symptoms and in whom the infarct vessel can be recanalized within 90 minutes of presentation. Primary PCI is also indicated for patients in whom thrombolytics are contraindicated and for patients in cardiogenic shock. Figure 12-4 shows angiograms of PCI for AMI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree