Subjects at risk of atherosclerosis might have dysfunctional high-density lipoprotein (HDL) despite normal cholesterol content in the plasma. We considered whether the efflux of excess cellular cholesterol to HDL from obese subjects is associated with impaired arterial endothelial function, a biomarker of cardiovascular risk. A total of 54 overweight (body mass index [BMI] 25 to 29.9 kg/m 2 ) or obese (BMI ≥30 kg/m 2 ) women, aged 46 ± 11 years, were enrolled in a worksite wellness program. The HDL cholesterol averaged 57 ± 17 mg/dl and was inversely associated with the BMI (r = −0.419, p = 0.002). Endothelial function was assessed using brachial artery flow-mediated dilation. Cholesterol efflux from 3 H-cholesterol–labeled baby hamster kidney cells transfected with the adenosine triphosphate-binding cassette transporter 1 showed 8.2% to 22.5% cholesterol efflux within 18 hours when incubated with 1% serum and was positively correlated with brachial artery flow-mediated dilation (p <0.05), especially in the 34 subjects with BMI ≥30 kg/m 2 (r = 0.482, p = 0.004). This relation was independent of age, HDL or low-density lipoprotein cholesterol concentrations in plasma, blood pressure, or insulin resistance on stepwise multiple regression analysis (β = 0.31, R 2 = 0.21, p = 0.007). Nitration of apolipoprotein A-I tyrosine residues (using sandwich enzyme-linked immunosorbent assay) was significantly greater in women with a BMI ≥30 kg/m 2 and the lowest cholesterol efflux than in women with a BMI of 25 to 29.9 kg/m 2 and the greatest cholesterol efflux (p = 0.01). In conclusion, we have shown that decreased cholesterol efflux by way of the adenosine triphosphate-binding cassette transporter 1 is associated with increased nitration of apolipoprotein A-I in HDL and is an independent predictor of impaired endothelial function in women with a BMI of ≥30 kg/m 2 . This finding suggests that the functional measures of HDL might be better markers for cardiovascular risk than the HDL cholesterol levels in this population.
It is widely accepted that the plasma concentrations of high-density lipoprotein (HDL) are inversely related to the risk of developing atherosclerotic vascular disease. One mechanism for vasculoprotection by HDL might be through facilitation of nitric oxide bioactivity in arterial endothelium, resulting in an overall benefit to vascular homeostasis. HDL-mediated reverse cholesterol transport, the mechanism by which excess cholesterol is effluxed from cells and transported to the liver, might also play a role in endothelial function. Cells other than macrophages express cholesterol efflux transporters, including endothelial cells. Thus, variation in HDL-mediated cholesterol efflux from endothelial cells or other cells in the vasculature might contribute to overall endothelial function, with the possibility of adverse effects in populations suspected of having “dysfunctional HDL” associated with obesity and diabetes.
Our objective was to measure the HDL cholesterol efflux capacity in women with HDL cholesterol levels generally within the normal range, but who are at risk of atherosclerosis because of obesity. Because endothelial cells and other cells in the vasculature express the adenosine triphosphate-binding cassette transporter 1 (ABCA1), we hypothesized that this property of HDL might show an association with endothelial function and thus provide insight regarding the role of HDL quality, despite adequate quantity, in vasculoprotection.
Methods
The present study was conducted at the Clinical Center of the National Institutes of Health with employees enrolled in a worksite wellness program initiated by the National Heart, Lung, and Blood Institute. The protocol, approved by the institutional review board of the National Heart, Lung, and Blood Institute ( NCT00666172 ), was open to women according to the body mass index (BMI; weight in kilograms divided by height in meters squared) classification of overweight (25 to 29.9 kg/m 2 ) or obese (≥30 kg/m 2 ), who were without a history of atherosclerotic vascular disease and were not participants in structured exercise or weight loss programs. All participants provided written informed consent to participate in the protocol. All subjects underwent focused cardiovascular physical examinations, and venous blood samples were drawn after an overnight fast. The standard lipid profiles were measured, using an enzymatic assay (Wako Chemical USA, Richmond, Virginia). Insulin resistance was estimated from the fasting glucose and insulin values using the homeostasis model assessment (HOMA). For women of reproductive age reporting menses, testing was performed during the follicular phase (days 1 to 10) of the menstrual cycle.
Brachial artery flow-mediated dilation testing, as an index of endothelial nitric oxide bioactivity, was conducted by a single investigator (G.Z.) as follows. Imaging of the left brachial artery proximal to the antecubital fossa was performed using a high-resolution ultrasound (12.5-MHz) linear-array transducer after 10 minutes of rest. The arterial diameter was measured in millimeters from the leading edge of the intimal–lumen interface of the near wall to the leading edge of the lumen–intimal interface of the far wall, coincident with the R wave on the electrocardiogram (end-diastole), at ≥6 sites and averaged. The maximum increase in brachial artery diameter was then measured during reactive hyperemia after 5 minutes of forearm ischemia caused by inflation of a blood pressure cuff on the forearm to suprasystolic pressure (225 mm Hg). The formula was as follows: brachial artery flow-mediated dilation (%) = (postischemia – baseline diameter)/baseline diameter × 100.
HDL-associated proteins apolipoprotein A-I (apoA-I) and apoA-II were measured using turbidimetric immunoassay (Wako Chemicals USA). The HDL subparticle preβ-1 was measured using an enzyme-linked immunosorbent assay (ELISA; Polymedco, Cortlandt Manor, New York). The subparticle HDL2b was measured electrophoretically, as previously described, using the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California), and is expressed as a percentage of the total HDL particle. The capacity of a serum specimen to accept cholesterol effluxed by ABCA1 was measured using a stably transfected baby hamster kidney cell line expressing the human ABCA1 transporter. Baby hamster kidney cells transfected with a hygromycin-resistant control plasmid were used as the control cell line. Cholesterol efflux was conducted at 37°C in cells labeled with 3 H-cholesterol for 24 hours, washed, and incubated for 18 hours with the subject’s whole serum at 1% concentration. The percentage of efflux specific to the ABCA1 transporter was calculated by subtracting the radioactive counts in the blank medium (α-minimal essential medium with 0.1% bovine serum albumin) from the radioactive counts in the presence of HDL and then dividing the result by the sum of the radioactive counts in the medium plus the cell fraction.
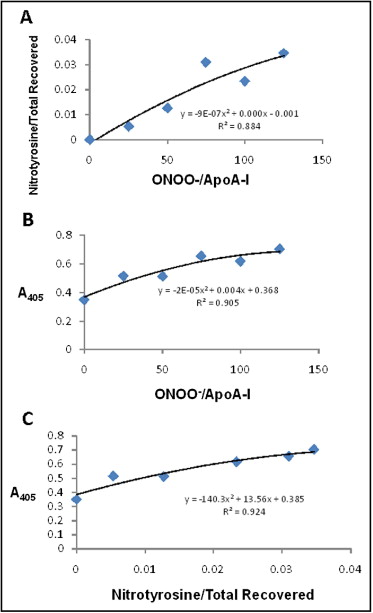
Nitrated apoA-I in the serum of the study participants with high and low efflux capacity was quantitated by a sandwich ELISA. In brief, sera from overweight and obese subjects with high and low cholesterol efflux were incubated in wells of a 96-well plate that was coated with anti-nitrotyrosine antibodies, enabling the capture of nitrated serum proteins, such as nitrated apoA-I. The plate wells were washed, and an antibody to human apoA-I was added, specifically identifying nitrated apoA-I. Serum with greater levels of nitrated apoA-I will have increased nitrated apoA-I bound to the well, resulting in greater levels of anti–apoA-I antibody binding. Horseradish peroxidase-conjugated secondary antibody followed by the horseradish peroxidase substrate OPD was used for detection. Absorbance at 405 nm was measured and corrected to the absorbance of the control wells in which no serum had been added. The assay was validated experimentally through the range of values detected in subject samples by adding increasing concentrations of peroxynitrite to purified apoA-I and either performing ELISA on the nitrated apoA-I or digesting the nitrated apoA-I with trypsin and using liquid chromatography–mass spectrometry to quantify tyrosine nitration of apoA-I. The number of apoA-I tryptic peptides containing nitrotyrosine, normalized to the total number of apoA-I tryptic peptides recovered, increased with increasing peroxynitrite dosage ( Figure 1 ). Between an absorbance (405 nm) of 0.3 and 0.7 U, the fit between the peroxynitrite dosage and absorbance (405 nm) from the ELISA assay (y = −2E-05x 2 + 0.004x + 0.368) was highly significant ( Figure 1 ). Finally, the number of apoA-I tryptic peptides containing nitrotyrosine, normalized to the total number of apoA-I tryptic peptides recovered, was correlated with absorbance (405 nm) in the ELISA assay ( Figure 1 ). Second-order polynomial equations and R 2 values were calculated using Microsoft Excel (Microsoft, Redmond, Washington).
The data are reported as the mean ± SD, unless otherwise indicated. All analyses were performed using Instat3 or Prism statistical software (GraphPad Software, San Diego, California) or SAS (SAS Institute, Cary, North Carolina). Simple linear regression analysis and the Pearson correlation coefficient were used to quantify the associations between the dependent and independent variables. Two-way analysis of variance with interaction was performed to determine the effects of cholesterol efflux and HDL cholesterol on brachial artery flow-mediated dilation. Multiple regression models for explaining brachial artery flow-mediated dilation were constructed, using stepwise model-building approaches by entering cholesterol efflux, age, HDL cholesterol, low-density lipoprotein cholesterol, triglycerides, blood pressure, and HOMA as covariates using the SAS statistical analysis package and the STEPWISE, SQUARE, GLM, and MEANS procedures (SAS User’s Guide, Statistics, version 9, SAS Institute). The statistical significance of differences in apoA-I tyrosine nitration were assessed using the unpaired t test. Both high and low efflux groups passed normality testing (Prism); p <0.05 was considered statistically significant.
Results
A total of 54 consecutive women meeting the eligibility criteria (age range 26 to 66 years) were enrolled during the initial year of the protocol and were evaluated in the present study. Of the 54 women, 20 were identified as overweight (BMI 25 to 29.9 kg/m 2 ) and 34 as obese (BMI ≥30 kg/m 2 ; Table 1 ). Of the 54 women, 7 (6 with a BMI of ≥30 kg/m 2 and 1 with a BMI of 25 to 29.9 kg/m 2 ) had adult-onset diabetes. Of these 7, 6 were taking oral hypoglycemic medications and 1 required insulin. For the remainder with normal fasting blood glucose levels, the insulin sensitivity was reduced (greater HOMA value) in the obese than in the overweight women. Also, 5 women with a BMI of ≥30 kg/m 2 and 3 with a BMI of 25 to 29.9 kg/m 2 were receiving statin therapy for lipid management. Finally, 3 subjects smoked cigarettes.
| Variable | BMI (kg/m 2 ) | ||
|---|---|---|---|
| 25–29.9 (n = 20) | ≥30 (n = 34) | p Value | |
| Age (years) | 49 ± 11 | 45 ± 11 | 0.1866 |
| European American | 13 (65%) | 15 (44%) | 0.167 |
| African American | 7 (35%) | 19 (56%) | |
| Waist circumference (cm) | 98 ± 8 | 116 ± 14 | <0.0001 |
| Homeostasis model assessment for insulin sensitivity | 1.7 ± 1 | 3.1 ± 2.1 | 0.0057 |
| Total cholesterol (mg/dl) | 203 ± 31 | 186 ± 32 | 0.0593 |
| Low-density lipoprotein cholesterol (mg/dl) | 118 ± 22 | 114 ± 33 | 0.5924 |
| High-density lipoprotein cholesterol (mg/dl) | 66 ± 18 | 52 ± 15 | 0.0042 |
| Triglycerides (mg/dl) | 81 ± 41 | 96 ± 46 | 0.1279 |
| Apolipoprotein A-I (mg/dl) | 150 ± 37 | 141 ± 28 | 0.38 |
| Apolipoprotein A-II (mg/dl) | 34 ± 8 | 33 ± 6 | 0.8516 |
| Preβ-1 (mg/dl) | 53 ± 25 | 42 ± 15 | 0.0618 |
| High-density lipoprotein 2b (%) | 27.7 ± 6.3 | 23.1 ± 5.1 | 0.0053 |
| Flow-mediated dilation (%) | 7.4 ± 3.5 | 6.5 ± 3.5 | 0.3527 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


