Chapter 44 Hiatal Hernia and Gastroesophageal Reflux Disease
Gastroesophageal Reflux Disease
Pathophysiology
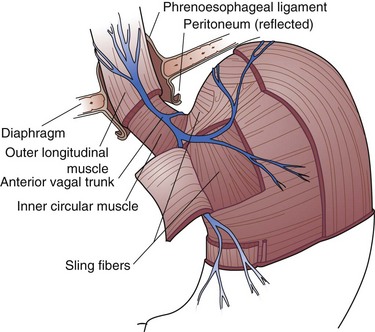
Several factors contribute to the generation of this high-pressure zone. The first is the intrinsic musculature of the distal esophagus. These muscle fibers differ from those in other areas of the esophagus in that they are in a state of tonic contraction. They normally relax with initiation of a swallow and then return to a state of tonic contraction. The second contributing factor to LES pressure is the sling fibers of the cardia. These fibers are at the same anatomic depth as the circular muscle fibers of the esophagus but are oriented in a different direction. They run diagonally from the cardia-fundus junction to the lesser curve (Fig. 44-1). These fibers are responsible for a significant percentage of the lower esophageal high-pressure zone. The third contributing factor to the maintenance of the high-pressure zone in the distal esophagus is the diaphragm. As the esophagus passes from the chest to the abdomen, it is surrounded by the crura of the diaphragm. During inspiration, the anteroposterior diameter of the crural opening is decreased, compressing the esophagus and increasing the measured pressure at the LES. This concept is particularly important for the interpretation of esophageal manometry tracings. By convention, assess the LES pressure at mid or end expiration, thereby providing reliable, reproducible pressure measurements. The last component of the pressure generated at the lower esophageal high-pressure zone is the transmitted pressure of the abdominal cavity. The abdominal compartment has a relatively higher pressure than the thoracic cavity. A GEJ that is firmly anchored in the abdominal cavity will be exposed to a greater transmural pressure than one that is in the posterior mediastinum.
Gastroesophageal reflux may occur when the pressure of the high-pressure zone in the distal esophagus is too low to prevent gastric contents from entering the esophagus or when a sphincter with normal pressure undergoes spontaneous relaxation, not associated with a peristaltic wave in the body of the esophagus.1 Other changes in the high-pressure zone, such as shortening, which occurs as part of cephalad displacement or as gastric distention from food or air, may also eliminate the barrier and result in reflux. Because even small changes in the high-pressure zone compromise its effectiveness, reflux episodes occur in normal people. The distinction between gastroesophageal reflux disease (GERD) and gastroesophageal reflux is a fine and important one and requires knowledge of associated symptoms, mucosal damage of the esophagus, total amount of acid exposure, and other factors.
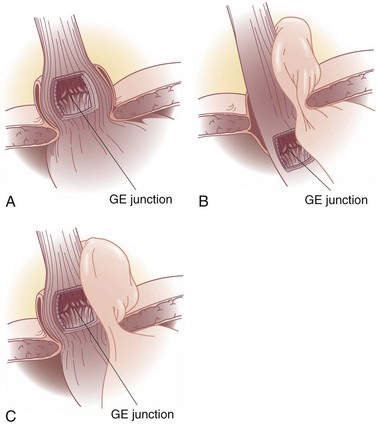
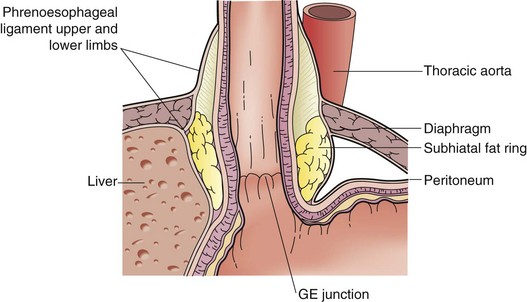
GERD is often associated with a hiatal hernia. Although any type of hiatal hernia may give rise to an incompetent cardia, the most common is the type I hernia (Fig. 44-2A), also called a sliding hiatal hernia. A type I hernia is present when the GEJ is not maintained in the abdominal cavity by the phrenoesophageal ligament (membrane). Thus, the cardia migrates back and forth between the posterior mediastinum and peritoneal cavity. The phrenoesophageal ligament is a continuation of the endoabdominal fascia, which reflects onto the esophagus at the hiatus. It lies just superficial to the peritoneal reflection at the hiatus and continues into the mediastinum (Fig. 44-3). Although the presence of a small sliding hernia does not necessarily imply an incompetent cardia, the larger its size, the greater the risk for abnormal gastroesophageal reflux.
Hiatal hernias are classified by their anatomy into three types (I to III). Types II and III hiatal hernias are often referred to as paraesophageal hernias and, although they may be associated with GERD, are also larger, more difficult hernias to treat and may be associated with acute or chronic obstructive symptoms. A type II hernia (see Fig. 44-2B), also called a rolling or paraesophageal hernia, occurs when the GEJ is anchored in the abdomen but the hiatal defect, which is usually large, provides space for viscera to migrate into the mediastinum. The relatively negative pressure in the thorax facilitates visceral migration. Usually, the fundus of the stomach migrates into the mediastinum; however, the colon and spleen are also occasionally identified. This is discussed in more detail later in this chapter (“Paraesophageal Hernias”). A type III hernia (see Fig. 44-2C) is a combination of the first two, in which the GEJ and fundus (or other viscera) are free to move into the mediastinum.
Clinical Presentation
Other symptoms may be present in patients with gastroesophageal reflux. Most arise from the gastrointestinal tract; however, many patients will have symptoms involving the respiratory tract as well, called extraesophageal symptoms. The frequency of symptoms in more than 1000 patients evaluated at the gastrointestinal function laboratory of the University of Washington is shown in Table 44-1. Although many patients with gastrointestinal symptoms will also complain of extraesophageal symptoms, it is less common for a patient to present with only respiratory symptoms. This is discussed in detail at the end of this section.2
Table 44-1 Prevalence of Symptoms in Gastroesophageal Reflux Disease*
| SYMPTOM | PREDOMINANCE (%) |
|---|---|
| Heartburn | 80 |
| Regurgitation | 54 |
| Abdominal pain | 29 |
| Cough | 27 |
| Dysphagia for solids | 23 |
| Hoarseness | 21 |
| Belching | 15 |
| Bloating | 15 |
| Aspiration | 14 |
| Wheezing | 7 |
| Globus | 4 |
* In more than 1000 patients evaluated. Symptoms reported occurred more frequently than once a week.
Preoperative Evaluation
Endoscopy
The endoscope has been used to grade the so-called flap valve.3 This is interpreted on a retroflexed view of the GEJ. The flap valve is graded from 1 to 4, with 4 being a completely patulous junction, with the lumen of the esophagus in full view from the body of the stomach.
Manometry
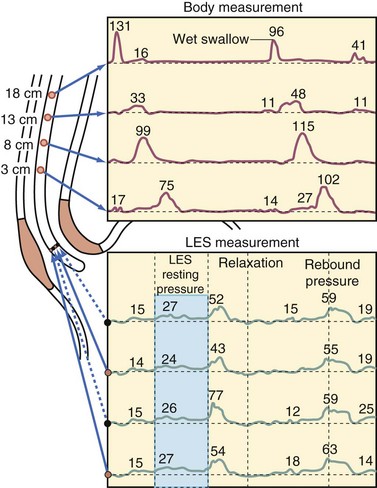
A significant amount of information about the function of the esophageal body and LES may be obtained from stationary esophageal manometry. This test will allow the surgeon to rule out primary motility disorders such as achalasia, which may mimic the symptoms of reflux and, in patients with GERD, will allow the surgeon to plan the operative procedure better by providing data about the ability of the esophagus to clear itself of ingested food. The manometry catheter is a flexible tube with pressure-sensing devices (water, perfused, or solid state) arranged at 5-cm intervals (Fig. 44-4). The upper esophageal sphincter (UES) is notoriously difficult to analyze because it migrates during the cervical phase of swallowing. Fortunately, the characteristics of the UES are infrequently relevant to clinical practice. The pertinent information to be gained from the manometry tracings concerns the function of the LES and the esophageal body.
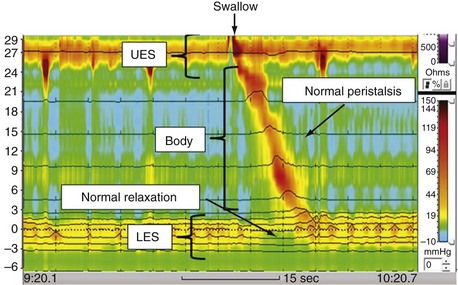
High-resolution manometry is now being used to characterize esophageal function more accurately as compared with standard manometry. The specific advantage of high-resolution manometry is that it allows for effective continuous recording of motor activity along the entire length of the esophagus and yields a more complete and detailed picture of esophageal motility. A color-contour plot with time as the x-axis and esophageal length as the y-axis is produced by the recording device. Pressure is represented by a color scale (Fig. 44-5). This method also provides a more detailed analysis of the LES and is less likely to show a decrease in LES pressure with deglutition, sometimes referred to as pseudorelaxation.
pH Monitoring
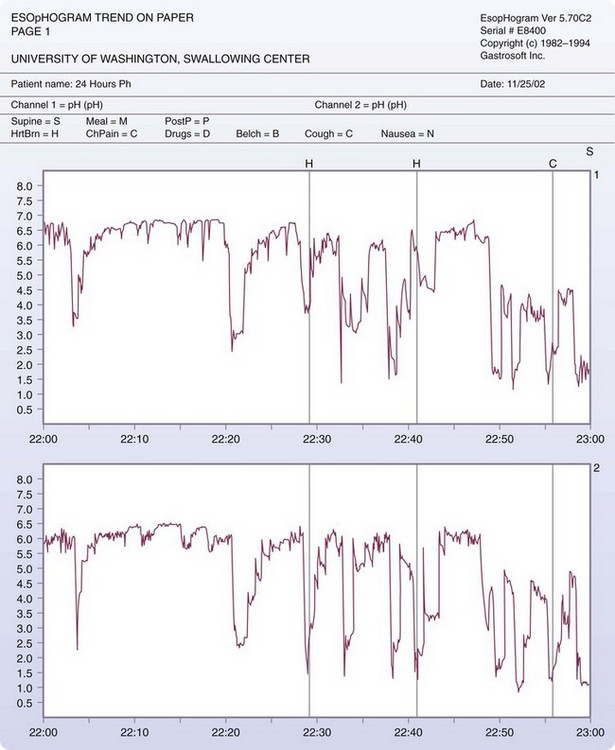
The gold standard for diagnosing and quantifying acid reflux is the 24-hour pH test. This study is performed by placing a thin catheter containing one or more solid-state electrodes in the esophagus. The electrodes are spaced 5 to 10 cm apart and are capable of sensing fluctuations in the pH between 2 and 7. The electrodes are connected to a data recorder that the patient wears for the period of observation. There is a digital clock displayed on the recorder. When the patient has an event (e.g., heartburn, chest pain, eructation), he or she records the event in a diary, noting the time on the recorder (Fig. 44-6).
Impedance pH testing is now being performed at specialized centers; this allows for further characterization of the refluxate as being acidic or nonacidic. Impedance monitoring detects reflux events based on the change in resistance to flow of an electrical current between electrodes, regardless of whether the refluxate is gas, liquid, or mixed. The intraluminal impedance increases with air and decreases with a liquid bolus. As compared with standard pH monitoring, impedance pH testing can distinguish between a true reflux event and ingestion of an acidic beverage by characterizing retrograde movement, as opposed to antegrade (normal swallow) along the esophagus. Also, another advantage is the ability of impedance pH testing to determine the proximal extent of reflux, which may be particularly useful for patients with extraesophageal symptoms such as cough, hoarseness, wheezing, or aspiration. One disadvantage, however, is that the automated analytic software tends to overestimate the number of reflux episodes, making it mandatory for all studies to be personally reviewed and edited manually, which can be time-consuming. Several studies have revealed that 30% to 40% of patients with persistent symptoms, despite maximal proton pump inhibitor (PPI) therapy with normal distal acid exposure as assessed by standard pH monitoring, have non–acid reflux events, with a high symptom correlation.4,5 These persistent complaints tend to be regurgitation, chest pain, cough and, much less frequently heartburn. Currently, it remains unclear how impedance pH testing should be implemented in the clinical management of GERD. Until more research has been carried out, it should only be performed at specialized esophageal centers and in select patients. It tends to have the highest yield in patients with atypical symptoms of GERD or in patients with pulmonary symptoms thought to be related to or exacerbated by proximal esophageal reflux events.6
Esophagography
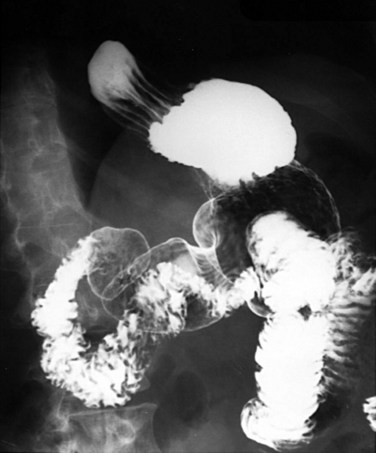
The esophagogram provides valuable information in the evaluation of patients with symptoms of GERD when an operation is contemplated or when the symptoms do not respond as expected. Often, spontaneous reflux during the examination will be demonstrated. Although reflux may be induced in patients who do not have the disease, the occurrence of spontaneous reflux lends support to the diagnosis of abnormal gastroesophageal reflux. The true value of the study is to determine the external anatomy of the esophagus and proximal stomach. The presence and size of a hiatal hernia may be characterized (Fig. 44-7). Although this neither confirms nor refutes the presence of disease, it is extremely beneficial in planning the operation. A mediastinal GEJ that does not reduce into the peritoneal cavity during the study is a predictor of a more difficult operation that may require an esophageal lengthening procedure. Peptic esophageal strictures may also be found on an esophageal contrast study. The presence of a stricture will taint the interpretation of the 24-hour pH study, especially if it is tight enough to prevent reflux. Other anatomic abnormalities, such as diverticula, tumors, and unexpected paraesophageal hernias, will be discovered during esophagography. Esophagograms are being replaced by CT scans, which, when reconstructed, provide all the information of the esophagogram but have the benefit of providing information about the other adjacent organs.
Other Tests
In unique circumstances, other diagnostic tests may be valuable. Occasionally, a patient will not be able to tolerate nasoesophageal intubation. A scintigraphic study to evaluate esophageal clearance and reflux may provide evidence of motility disorder and gastroesophageal reflux.7 Gastric distention from delayed emptying may also be diagnosed with a scintigraphic study. Although this condition may contribute to reflux, it is not clear whether a gastric emptying procedure (pyloroplasty) needs to be added to an antireflux procedure in a patient with delayed gastric emptying.
Treatment and Outcome
Medical Management
When a patient is first seen, a lengthy workup is not necessary if the history and examination are consistent with GERD. It would be prudent to check for chronic anemia in such a patient and to prescribe a 6-week course of acid suppression therapy. Most authors agree that a double dose of a PPI is the initial approach to medical management. Given in this manner, the use of medical therapy becomes in itself a diagnostic tool. If the symptoms persist after a trial of medical therapy, a more extensive evaluation, as described earlier, would be indicated. The medications available to treat acid reflux include antacids, motility agents, histamine 2 (H2) blockers, and PPIs. Although lifestyle modification has been advocated before or as an adjunct to medical therapy, the efficacy of such changes in the treatment of esophagitis has not been proved.8
Pharmacologic treatment of GERD has been revolutionized by the advent of PPIs. This class of drugs is one of the most widely prescribed drugs worldwide and, in 2006, the expenditure on these drugs was approximately $24 billion.9 These drugs act by irreversibly binding the proton pump in the parietal cells of the stomach, thus effectively stopping gastric acid production. The maximal effect occurs after approximately 4 days of therapy and the effects linger for the life of the parietal cell. Thus, the acid suppression will persist for 4 to 5 days after therapy has ended so the patient needs to be off therapy for 1 week before being evaluated with pH monitoring.
Compared with H2 blockers, PPIs are more effective at healing esophageal ulceration secondary to acid exposure.10 These medications are relatively expensive but are well tolerated. Side effects may include headache, abdominal pain, flatulence, constipation, and diarrhea. Recently, long-term side effects of PPI therapy have gained significant attention. Several studies have revealed an association between long-term PPI use and increased risk of nutritional deficiencies and infectious complications.9 The results of these studies, however, need to be interpreted with caution, given that most were limited by small sample size and retrospective design. Larger studies have linked long-term use of PPIs to gastric polyp formation, usually occurring with more than 1 year of treatment. Most of these polyps are hyperplastic and do not appear to be malignant.
Surgery
Since the application of minimally invasive techniques to the treatment of GERD, the cost of surgery has decreased. This has changed how surgical treatment is viewed. Considering the cost of PPI use and the cost of operative treatment with its accepted success rate, the length of time required for medical therapy to become more expensive than surgery is approximately 8 to 10 years.11 This assumes that the patient uses the lowest dose of the medication. Therefore, in patients who have more than 8 years of life expectancy and are in need of lifelong therapy because of a mechanically defective sphincter, surgical therapy may be considered the treatment of choice.
360-Degree Wrap (Left Crus Approach)
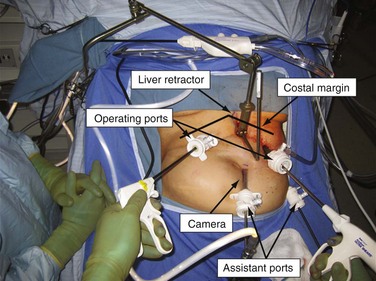
The patient is placed in a low lithotomy position. The surgeon stands between the patient’s legs, with the assistant on the left side of the patient. The four trocars and liver retractor are placed so that two equilateral triangles sharing a common medial angle are created. The surgeon operates through the two most cephalad ports. The assistant operates through the two closest caudad ports. The liver retractor is placed just left of the midline, in the subxiphoid region (Fig. 44-8).
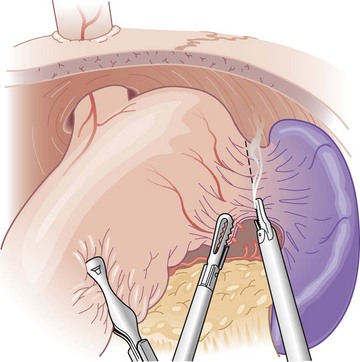
With the assistant first retracting the greater curve and then the omentum, the left crus and greater curve are dissected by the surgeon. The short gastric vessels are taken early to mobilize the fundus (Fig. 44-9). With the fundus mobilized, the phrenoesophageal membrane over the left crus may be dissected until the crural fibers are identified. The entire length of the left crus is mobilized at this time (Fig. 44-10).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree