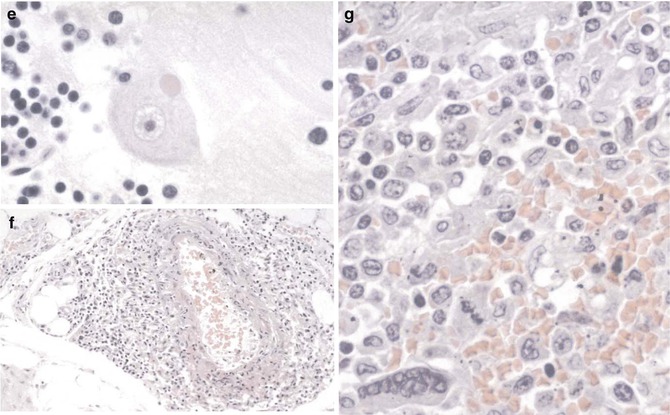
Fig. 14.1
Nipah virus. (a) Pulmonary vessels showing mild vasculitis and a multinucleated endothelial syncytium. (b) Interstitial pneumonitis. Note small vessel lined by a multinucleated endothelial cell. (c) Interstitial pneumonitis with intra-alveolar multinucleated giant cells containing nuclear inclusions. (d) Viral nature of giant cells, in (c), as evidenced by immunostaining for Nipah viral antigens. (e) Typical eosinophilic viral inclusion (“Negri-like”) in cytoplasm of a neuron. (f) Moderate vasculitis in soft tissue in a fatal case of Nipah virus infection. (g) Multinucleated giant cell as seen in the splenic parenchyma. In the lung, giant cells can also be seen in other organs such as kidney and lymphoid. (a)–(c), (e)–(g), hematoxylin and eosin; (d), immunoalkaline phosphatase staining, naphthol fast red, and hematoxylin counterstain (Courtesy of Dr. S.R. Zaki, Centers for Disease Control and Prevention, Atlanta, GA; from Dail and Hammar’s Pulmonary Pathology, 3rd ed, Ch 11 Viral Infections of the Lung, by Tomashefski, with kind permission of Springer Science + Business Media)
14.8 Diagnosis
The diagnosis of Nipah virus infection, suspected by patient history and clinical manifestations, can be supported by characteristic histopathologic findings. From a diagnostic standpoint, perhaps the most unique histopathologic finding is the presence of syncytial and parenchymal multinucleated endothelial cells. This feature, however, occurs in only approximately one fourth of the cases and cannot be used as a sensitive criterion for the diagnosis of Henipah virus infections. Moreover, these cells can also be seen in measles virus, respiratory syncytial virus, herpesviruses, and other infections. Unequivocal diagnosis can be made only by other laboratory tests such as immunohistochemistry, cell culture isolation, polymerase chain reaction, or serology (Zaki and Paddock 2008).
Nipah virus infection can be diagnosed by a variety of serological, microbiological, and immunofluorescent techniques and other methods such as the PCR assay. Through screening of hybridomas derived from mice immunized with γ-irradiated Nipah virus, Chiang et al. identified two secreted antibodies, one reactive with the nucleocapsid (N) protein and the other reactive with the phosphoprotein (P) of henipaviruses. Epitope mapping and protein sequence alignments between NiV and HeV suggested the last 14 amino acids of the carboxyl terminus of the N protein is the target of the anti-N antibody. The anti-P antibody recognizes an epitope in the amino-terminal half of P protein. These monoclonal antibodies were used to develop two antigen-capture enzyme-linked immunosorbent assay (ELISA) tests, one for virus detection and the other for differentiation between NiV and HeV. The lower limit of detection of the capture assay with both monoclonal antibodies was 400 pfu. The anti-N antibody was used to successfully detect NiV in a lung tissue suspension from an infected pig (Chiang et al 2010). The findings further suggested that antigen-capture ELISA testing is a potentially affordable tool to provide rapid detection and differentiation of the Henipah virus.
14.9 Differential Diagnosis
Clinical findings including pulmonary and extrapulmonary manifestations in febrile western patients with history of travel abroad, particularly Australia, Malaysia, Singapore, and Bangladesh may lead to suspect Henipah virus infection. Histologically, the presence of syncytial parenchymal multinucleated endothelial cells is important but is known to occur in only one fourth of the patients. Moreover, multinucleated cells can be seen in patients with other viral infections such as measles, respiratory syncytial virus, and herpetic infections and are therefore nondiagnostic but still useful helping to raise suspicion.
14.10 Vaccine
Walpita et al. studied the vaccine potential of NiV virus-like particles (NiV VLPs) composed of three NiV proteins, G, F, and M. Co-expression of these proteins under optimized conditions resulted in quantifiable amounts of VLPs with many virus-like/vaccine-desirable properties including some not previously described for VLPs of any paramyxovirus (Walpita et al. 2011): The particles were fusogenic, inducing syncytial formation; PCR array analysis showed NiV VLP-induced activation of innate immune defense pathways; the surface structure of NiV VLPs imaged by cryoelectron microscopy was dense, ordered, and repetitive, and consistent with a similarly derived structure of the paramyxovirus measles virus. The VLPs were composed of all the three viral proteins as designed, and their intracellular processing also appeared similar to NiV virions. The size, morphology, and surface composition of the VLPs were consistent with the parental virus, and, importantly, they retained their antigenic potential. Finally, these particles, formulated without adjuvant, were able to induce a neutralizing antibody response in B/c mice. The authors concluded their findings indicate vaccine potential, while suggesting the need for ongoing research in animal models of Nipah virus disease (Walpita et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


