Hemostatic Devices
James E. Harvey
Michael A. Lincoff
Obtaining hemostasis in patients after cardiovascular catheterization is a critical component of the procedure. When the novel technique for percutaneous endovascular access was introduced by Seldinger over 50 years ago, his reported method for achieving hemostasis included “20 to 30 minutes of hand-held pressure after catheter removal followed by overnight bed rest.” Since then, manual pressure or mechanical compression has remained the gold standard for achieving postprocedural hemostasis following femoral artery puncture. However, with the increase in coronary and peripheral vascular procedures over the past two decades has come a demand for more efficient and cost-effective methods of achieving hemostasis. This has led to the development of many types of vascular closure devices (VCDs) designed to increase patient comfort while maintaining safety and ease-of-use. These devices offer the advantages of early sheath removal, early ambulation, and early hospital discharge, as well as allow for uninterrupted anticoagulation when needed. In addition to manual compression or mechanical compression devices (including mechanical clamp devices and hemostatic pads), the currently available VCDs fall into three major categories: collagen-based biosealant, percutaneous suture based, and staples and clips, with minimal variation in cost between the devices ($175 to $190 at our institution).
Devices
Manual Compression:
Despite many available VCDs on the market, manual pressure remains a fundamental component of arteriotomy management because of its low cost, good safety profile (complication rate of 0.23% following diagnostic catheterization in large series and >50 years experience), short learning curve, and ability to be employed despite femoral artery dissection, significant peripheral vascular disease, or a “low stick.” Limitations of this technique include the length of time needed before ambulation, prolonged hospitalization time, need for
trained personnel, patient discomfort, staff fatigue, and slowing of the catheterization laboratory workflow. For patients following percutaneous coronary intervention (PCI) or in those who have been on anticoagulation with heparin, manual compression can be safely performed once the activated clotting time (ACT) is less than 180 seconds or the activated partial thromboplastin time (aPTT) is less than 50 seconds.
trained personnel, patient discomfort, staff fatigue, and slowing of the catheterization laboratory workflow. For patients following percutaneous coronary intervention (PCI) or in those who have been on anticoagulation with heparin, manual compression can be safely performed once the activated clotting time (ACT) is less than 180 seconds or the activated partial thromboplastin time (aPTT) is less than 50 seconds.
When removing the sheath, gentle pressure is applied over the skin puncture site being careful not to crush the sheath and “strip” clot into the femoral artery. Manual pressure is then held directly above the arteriotomy at a point approximately 1.5 cm cephalad to the skin puncture site. Pressure should be held for approximately 3 minutes per French size for arterial punctures and 2 minutes per French size for venous punctures and can be gradually reduced over that time (i.e., after a 6-Fr. arterial sheath removal, hold full pressure for 5 minutes, then 75% pressure for 5 minutes, then 50% pressure for 5 minutes, then 25% pressure for 3 to 5 minutes). The pedal pulses should be checked every few minutes during femoral artery compression. If the pedal pulses are absent during femoral artery compression, the pressure should be intermittently reduced to allow perfusion to the distal lower extremity. Keep in mind that this is only an estimate and that patients with a mildly elevated ACT or those receiving antiplatelet therapy (i.e., aspirin, clopidogrel, prasugrel, etc.) may need an additional 10 to 15 minutes of manual pressure to achieve hemostasis. Low-risk patients should remain supine for 2 to 3 hours (0.5 hours per French size) after hemostasis, especially when a small diameter catheter is used (≤5 Fr.); high-risk patients should remain supine for 4 to 6 hours after hemostasis. One study involving low-risk patients (5-Fr. sheath, diagnostic catheterization only, no anticoagulation) who received 10 to 15 minutes of manual compression followed by 1 hour of bed rest and 1 hour of observation reported a minor complication rate of 3.3% and a major complication rate of 0.1%. The major complication of manual compression is pseudoaneurysm that results from poor hemostasis (pressure).
Hemostatic Pads:
Hemostatic pads (D-Stat Dry, SyvekPatch, Chito-Seal) are small pieces of gauze or other material that are impregnated with a procoagulant mixture that causes local vasoconstriction and potentiates clot formation. Used in conjunction with manual compression, the patch is applied topically over the puncture site after sheath removal and remotely activates the coagulation cascade from the skin surface. Coagulation cascade activation is potentiated down the sheath tract to the arterial wall. The benefits of patches include decreased time to hemostasis, reduced time to ambulation, no insertion of foreign material into the body, and
immediate repeat arterial puncture if necessary. Other advantages include shortened hospitalization and a low incidence of major vascular complications (0.1%).
immediate repeat arterial puncture if necessary. Other advantages include shortened hospitalization and a low incidence of major vascular complications (0.1%).
D-Stat Dry hemostatic bandage (Vascular Solutions, Inc., Minneapolis, MN) is a nonwoven gauze patch that is coated with thrombin that potentiates the coagulation cascade by directly converting fibrinogen to fibrin. It is used as an adjunct to manual compression and is indicated to reduce the time to hemostasis in patients undergoing diagnostic endovascular procedures utilizing 4- to 6-Fr. sheaths. A recent study of 367 patients looked at the use of D-Stat Dry versus manual compression alone and found that use of D-Stat Dry resulted in a significant reduction in time to hemostasis (7.8 vs. 13.0 min; p = 0.001) with no significant difference in major or minor complications. The D-Stat Dry patch is directly applied over the skin puncture site and manually compressed for a minimum of 6 minutes (for low-risk normotensive patients not anticoagulated) to 10 minutes (anticoagulated, hypertensive, large sheath size) or until hemostasis is achieved. ACT should be less than 180 seconds or in accordance with the local institutional guidelines. Not anticoagulated patients should not ambulate for 1.5 (4 Fr.) to 2.5 (6 Fr.) hours; anticoagulated patients should not ambulate for 2 to 4 hours. D-Stat Dry is contraindicated in patients with known sensitivity to bovinederived materials.
SyvekPatch (Marine Polymer Technologies, Inc., Danvers, MA) is made of poly-N-acetyl glucosamine (p-G1cNAc), which causes local vasoconstriction and potentiates clot formation. This small patch should be applied directly over the arterial puncture site and manually compressed for 10 minutes following sheath extraction. ACT should be less than 300 seconds. Patients are required to lie supine for 2 hours after the patch has been held in place. An uncontrolled study of 200 patients who underwent diagnostic coronary angiography with a 6-Fr. catheter evaluated the use of the SyvekPatch as an adjunct to manual pressure followed by 1 hour of bed rest reported no major and only 2% minor adverse events, all of which were successfully managed with additional 1 to 2 hours of bed rest.
Mechanical Compression:
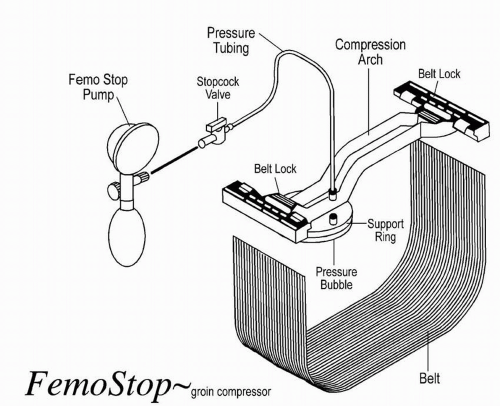
Mechanical compression involves the use of a C-arm clamp, sandbags, or a pneumatic compression device (FemStop). The C-arm clamp is a device with a flat base and a horizontal arm that extends over the base and angles down at a 90° angle to apply pressure to the femoral artery. The tip of the device consists of a metal or plastic disk, which is placed directly over the arterial puncture site for approximately 20 to 30 minutes. The FemStop applies direct
pneumatic pressure over the femoral artery to tamponade bleeding. A transparent plastic bubble is placed over the arterial puncture site and secured with a plastic arch and belt wrapped around the patient (Figure 9-1).
pneumatic pressure over the femoral artery to tamponade bleeding. A transparent plastic bubble is placed over the arterial puncture site and secured with a plastic arch and belt wrapped around the patient (Figure 9-1).
A recent study that compared manual to mechanical compression demonstrated that time to hemostasis was approximately 33% shorter with manual compression. Studies done in the 1970s and 1980s noted that there were no significant differences in rates of vascular complications between manual and mechanical compression techniques. Although mechanical compression devices provide a hands-free approach, they do not eliminate the need for staff supervision during the period of compression.
Collagen-based Biosealant Devices:
Collagen-based biosealant VCDs utilize bovine collagen-based products to facilitate clot formation. These VCDs augment hemostasis via two mechanisms: (1) the device deploys a collagen mass that expands after deployment and mechanically seals the arterial wall and sheath tract and (2) it provides additional collagen to the arterial wall defect promoting platelet adherence, activation, and aggregation.
Collagen-based VCDs are generally used in patients who are unlikely to require immediate repeat arterial access. Due to the expansive collagen product, it may be difficult to reaccess the artery close to the previous puncture site and it is usually recommended to wait 90 days until repeat puncture at the same site is attempted. Collagen-based devices should only be used when arterial access was obtained with a single anterior puncture of the common femoral artery. Several different models of collagen-based VCDs are commercially available; the three most common are Angio-Seal, DuettPro, and VasoSeal.
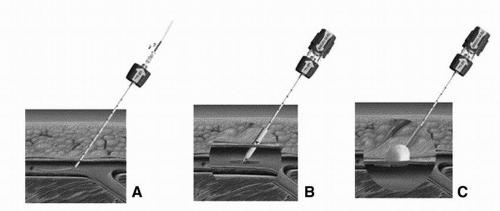
Angio-Seal (St. Jude Medical, St. Paul, Minn) is a popular collagen-based VCD that involves placing a collagen plug directly over an intravascular anchoring system. First, a carrier sheath is exchanged for the femoral artery sheath (Figure 9-2). Once inserted, the intravascular anchor protrudes from the end of the carrier into the femoral artery. The sheath and carrier are then removed, which pulls the intravascular anchor against the inside of the arterial wall. Tension is applied to the connecting suture, advancing the collagen plug down onto the outside of the arterial wall defect. The patient is required to remain in the supine position for 2 hours after the Angio-Seal has been deployed. Repeat arterial puncture should not be performed for a period of 90 days. With regards to safety and efficacy, a randomized trial comparing the Angio-Seal to manual pressure showed that time to hemostasis following angiography was significantly shorter in the Angio-Seal group (2.5 minutes) compared with manual pressure (15.3 minutes). This study also demonstrated
significantly fewer complications, such as bleeding and/or hematoma formation, for Angio-Seal patients receiving heparin. In general, the Angio-Seal device affords rapid deployment, earlier hospital discharge, and improved patient comfort following cardiac catheterization. It has also been used successfully used in radial, carotid, and subclavian arterial puncture sites, venous puncture sites, and even right ventricular perforation. It has the lowest reported vascular complication rates of all closure devices.
significantly fewer complications, such as bleeding and/or hematoma formation, for Angio-Seal patients receiving heparin. In general, the Angio-Seal device affords rapid deployment, earlier hospital discharge, and improved patient comfort following cardiac catheterization. It has also been used successfully used in radial, carotid, and subclavian arterial puncture sites, venous puncture sites, and even right ventricular perforation. It has the lowest reported vascular complication rates of all closure devices.
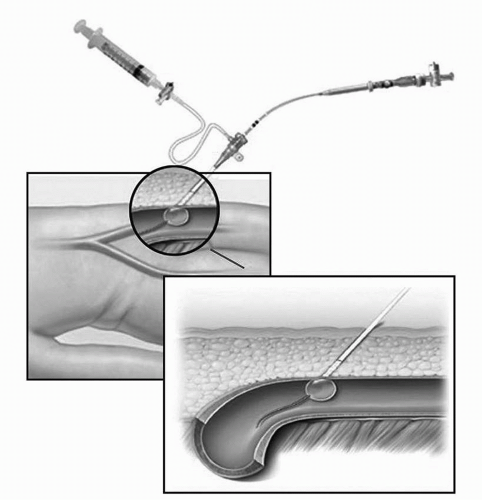
The DuettPro sealing device (Vascular Solutions, Minneapolis, MN) is a collagen-based VCD that utilizes a 7-mm balloon attached to a 3-Fr. catheter to inject a procoagulant mixture of collagen and thrombin (Figure 9-3). The device is advanced through the existing femoral sheath
into the arterial lumen, the mounted balloon is inflated in the artery, and then the entire device is gently retracted until the balloon abuts the arteriotomy puncture site. The mixture of collagen and thrombin is injected to the tissue surrounding the arterial puncture site. Thrombin in the presence of collagen converts fibrinogen to fibrin and accelerates the coagulation cascade. The sheath is removed, the balloon is deflated, and manual pressure is applied for 2 minutes. Patients are required to remain in the supine position for approximately 2 hours to promote adequate hemostasis and reduce the risk of complications. Unlike the Angio-Seal device, there is no contraindication to immediate repuncture with the Duett device. One serious potential complication of the Duett device is the inadvertent injection of the procoagulant collagen-thrombin mixture into the artery. A large study comparing the Duett device to manual pressure showed that the times to hemostasis and ambulation were significantly lower with the Duett device, but the incidence of major vascular complications was higher.
into the arterial lumen, the mounted balloon is inflated in the artery, and then the entire device is gently retracted until the balloon abuts the arteriotomy puncture site. The mixture of collagen and thrombin is injected to the tissue surrounding the arterial puncture site. Thrombin in the presence of collagen converts fibrinogen to fibrin and accelerates the coagulation cascade. The sheath is removed, the balloon is deflated, and manual pressure is applied for 2 minutes. Patients are required to remain in the supine position for approximately 2 hours to promote adequate hemostasis and reduce the risk of complications. Unlike the Angio-Seal device, there is no contraindication to immediate repuncture with the Duett device. One serious potential complication of the Duett device is the inadvertent injection of the procoagulant collagen-thrombin mixture into the artery. A large study comparing the Duett device to manual pressure showed that the times to hemostasis and ambulation were significantly lower with the Duett device, but the incidence of major vascular complications was higher.
The VasoSeal hemostatic devices (VasoSeal Elite and VasoSeal ES; Datascope, Montvale, NJ) are collagen-based VCDs that utilize a purified collagen plug to accentuate hemostasis. To deploy these devices, a dilator and a sheath are collectively advanced over a guidewire to the surface of the femoral artery (Figure 9-4). The dilator is removed and the collagen plug is advanced through the sheath into the vascular access track. Following placement of the device, patients are kept supine for 2 hours. This device is similar to the Angio-Seal device in that it delivers a collagen plug into the skin tract; however, there is no intraluminal component remaining after the device is deployed. In patients undergoing coronary angiography, the Vasoseal device had mean times to hemostasis and ambulation of 18 minutes and 110 minutes,
respectively; however, an increase in vascular complications following PCI has been noted.
respectively; however, an increase in vascular complications following PCI has been noted.
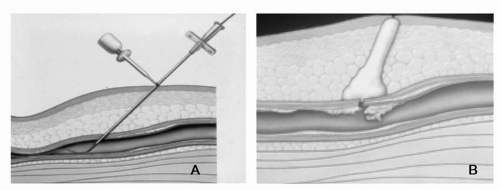 Figure 9-4 The VasoSeal closure device. A) Dilator advanced over guidewire to previously demarcated depth at surface of femoral artery. B) Collagen injected over arteriotomy site while simultaneously withdrawing delivery apparatus resulting in vascular hemostasis.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|