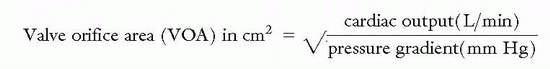Oximetry Measurements:
Oximetry measurements are most commonly performed to measure cardiac output utilizing the Fick method (described later in this chapter) and to rule out a left-to-right shunt (described later in this chapter). Oximetry measures the oxygen saturation of blood. The oxygen content of blood can then be calculated.
Oxygen content ˜Hgb(g/dL) × 1.36(mLO2/g Hgb) × Sat
where Hgb is hemoglobin in grams per deciliter; 1.36 is the oxygen carrying capacity of blood in milliliters of oxygen per gram of hemoglobin; and Sat is the oxygen saturation of the blood. The dissolved oxygen in blood is generally negligible in these calculations and is usually ignored.
PRESSURE GRADIENTS ACROSS STENOSES:
Measuring pressure gradients across stenotic valves is an important process in determining the need for surgical intervention particularly when the hemodynamics as measured by noninvasive means are in question. The valve orifice area can often be estimated by a formula that was developed by Dr. Richard Gorlin if the mean pressure gradient, cardiac output, and the systolic ejection time are known; particularly if the patient is not in a low cardiac output state:
where SEP is the systolic ejection period in aortic stenosis (length of time blood is ejected from LV every beat); DFP is the diastolic filling period in mitral stenosis (length of time blood filling LV every beat); ΔP is the mean pressure gradient; constant (K = 0.85) is added in mitral stenosis.
The Hakki formula is a simplified derivation of the Gorlin equation:
The Angel correction mandates that the above result be divided by 1.35 for a heart rate <75 beats per minute in the setting of mitral stenosis, or >90 beats per minute in the setting of aortic stenosis.
Caution is advised when using the Hakki formula if coexisting aortic regurgitation or mitral regurgitation is present as this will cause underestimation of the aortic valve area and mitral valve area respectively.
Aortic Stenosis: The normal orifice area of the aortic valve is 3 to 4 cm2. The aortic valve can become significantly narrowed prior to the onset of symptoms or even hemodynamic significance.
Aortic Valve Orifice Areas |
Normal aortic orifice area |
3-4 cm2 |
Mild stenosis |
>1.5 cm2 |
Moderate stenosis |
1.0-1.5 cm2 |
Severe stenosis |
<1.0 cm2 |
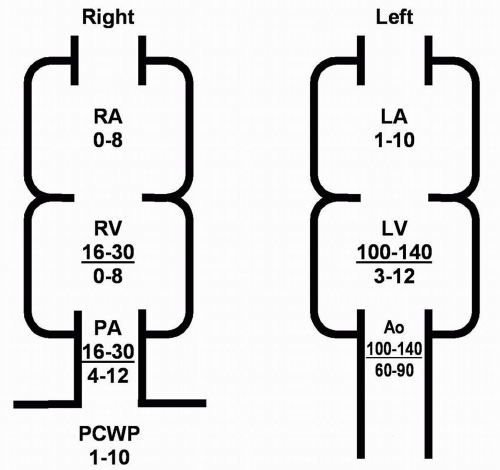
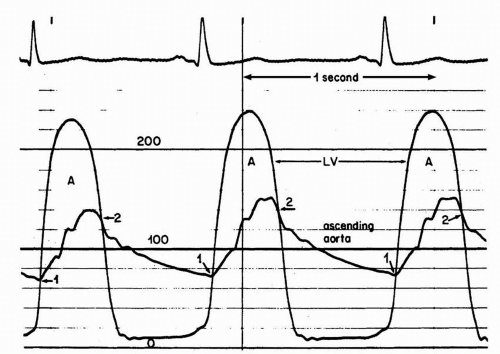
The most accurate method for measuring aortic valve gradients is by obtaining simultaneous pressure measurements from the left ventricle and the ascending aorta (Figure 7-7). This method allows the calculation of the mean gradient by direct measurement from both recordings. The easiest way to accomplish this is to use a dual-lumen pigtail catheter, which permits simultaneous measurement of pressures in the LV and ascending aorta.
Alternatively, a long arterial sheath can be placed in the descending thoracic aorta, and pressure measured from the sideport. The femoral artery pressure is also often substituted for this measurement. The peak femoral artery pressure is usually higher than the peak aortic root pressure due to reflected pressure waves seen in the periphery, thus using the femoral artery results in underestimation of the pressure gradient. This can be somewhat compensated by measuring the pressure difference between the catheter at the ascending aorta and the sidearm of the femoral artery sheath, and subtracting the difference.
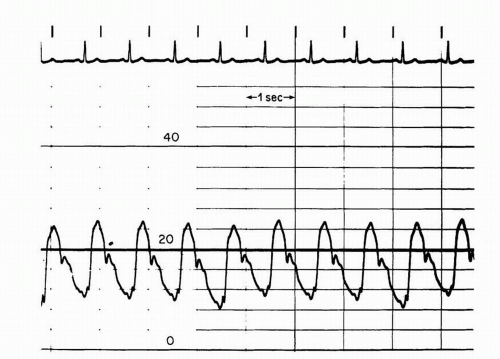
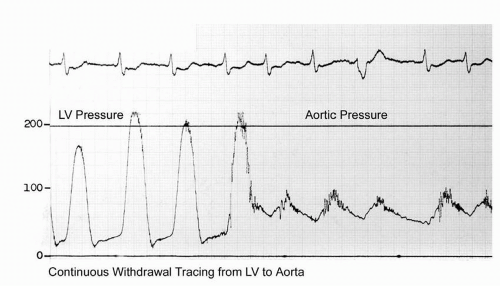
A more commonly utilized method involves pullback of the catheter from the left ventricle into the ascending aorta. This technique yields a “peak-to-peak” gradient between the maximum aortic pressure and the
maximum left ventricular pressure (
Figure 7-8). Each of these peaks occurs at different points in time, however, and this measurement is only an estimate of the mean gradient. In addition, in patients with severe aortic stenosis, the catheter itself may take up a significant fraction of the orifice area, resulting in worsened stenosis and increased gradients.
The Gorlin and Hakki formulas can be used to estimate the valve orifice area, but may be inaccurate in severe aortic stenosis with low-output states. The accuracy of the formula is flow-dependent and will result in small orifice areas, despite low gradients, if the flow across the aortic valve is low. This is frequently observed in patients with severe systolic LV dysfunction.
If maneuvers to increase cardiac output (i.e., exercise, dobutamine, nitroprusside) are performed on this subset of patients and a significant increase in the estimated valve orifice area is observed (usually resulting in a valve area >1 cm2) this is termed “pseudostenosis.” Failure of the estimated valve orifice area to significantly increase with these measures implies either true severe aortic stenosis (increase in aortic valve pressure gradients with maneuvers) or poor left ventricular contractile reserve (no significant increase in aortic valve pressures with maneuvers).
Mitral Stenosis: The normal mitral valve orifice area is 4 to 6 cm2. Significant narrowing can occur prior to hemodynamic compromise. When the valve area falls to ˜2.0 cm2, the left atrial pressures will start increasing to maintain cardiac output. Valve areas less than 1.0 cm2 frequently require some intervention.