Chapter 30 Hemoptysis Caused by Distal Left Main Bronchial Tumor in a Patient with Primary Lung Adenocarcinoma
Case Description
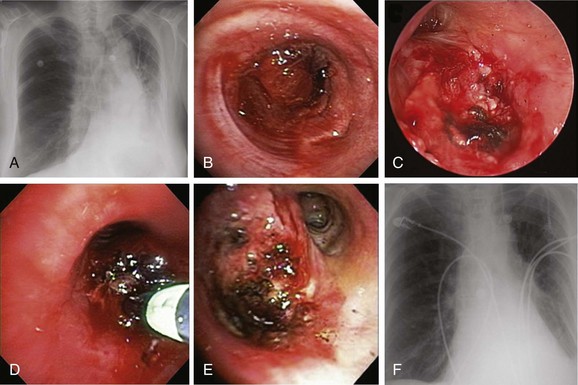
A 62-year-old female who had been diagnosed 6 weeks earlier with stage IV primary adenocarcinoma with rib and brain metastasis came to the emergency department with respiratory distress and hemoptysis. Hemoptysis was described as 4 to 6 tablespoons of bright red blood within 24 hours. She had a history of GERD and COPD requiring 2 L of home oxygen. She had smoked 1 pack per day for 20 years but quit 10 years earlier. At the time of her presentation, she had just completed a cycle of bevacizumab therapy and 10 days of brain radiotherapy. On physical examination, she was awake, alert, and oriented, without neurologic deficits; air entry in the left hemithorax was diminished. Her ECOG (Zubrod score)* before the hemoptysis was 1.1 Her heart rate was 110, blood pressure 160/90, and respiratory rate 28/min. She required 5 L of oxygen by nasal cannula to maintain oxygen saturation of 92%. Chest radiograph showed left-sided tracheal deviation and atelectasis of the left lower lobe and lingula. Aeration in the upper lung field zone was maintained (Figure 30-1, A). The patient consented to bronchoscopy with electrocautery; a large blood clot was noted completely occluding the distal left main bronchus (Figure 30-1, B). After clot removal, patency to the left upper lobe was restored, but active bleeding was noted coming from the tumor that was completely occluding the left lower lobe bronchus (Figure 30-1, C). The tumor was cauterized using an electrocautery probe (Figures 30-1, D and E). During the following hours, this controlled her hemoptysis. Follow-up chest radiograph post procedure shows improved aeration to the left upper lobe and persistent atelectasis in the left lower lobe (Figure 30-1, F).
Discussion Points
1. List two alternative bronchoscopic procedures that can be applied to control this patient’s hemoptysis.
2. List three contraindications to electrocautery performed through the flexible bronchoscope.
3. Describe and justify a diagnostic approach to a patient with lung cancer involving the mediastinum who presents with recurrent hemoptysis after bronchial artery embolization.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
This patient with underlying chronic obstructive pulmonary disease (COPD) and stage IV lung adenocarcinoma presented with respiratory distress likely exacerbated by the left main bronchial obstruction caused by tumor-related bleeding. Several definitions have been put forth for what “major” airway bleeding (hemoptysis) represents, as well as for quantifying the degree and amount of bleeding, but the actual practical and clinical implications of such definitions based on amounts of expectorated blood are unknown. According to one classification schema, bleeding is considered nonmassive for less than 600 mL/24 hr, massive for more than 600 mL/24 hr, exsanguinating for more than 1000 mL/24 hr or for a rate greater than 150 mL/hr, and catastrophic when it causes an immediate threat to life. Based on this system, our patient had a nonmassive hemoptysis (4 to 6 tablespoons is the equivalent of 60 to 90 mL). This is similar to what is considered a significant bronchoscopy-related bleeding in other classification schemas, defined in studies as more than 50 mL.*2 It appears that when it comes to hemoptysis, no uniform cutoff value is agreed upon in the literature to define “massive” (Table 30-1).3–10
Table 30-1 Definitions for “Massive” Hemoptysis Based on the Amount or Rate of Expectorated Blood
| Defining Terms | Amount or Rate of Expectorated blood | Reference |
|---|---|---|
| Massive hemoptysis | ≥200 mL/24 hr | 3 |
| Major hemoptysis | ≥200 mL/24 hr | 4, 5 |
| Severe hemoptysis | ≥150 mL/12 hr | 6 |
| Severe hemoptysis | >400 mL/24 hr | 7 |
| Exsanguinating hemoptysis | ≥1000 mL or ≥150 mL/hr | 8 |
| Life-threatening hemoptysis | ≥600 mL/24 hr | 9 |
| Life-threatening hemoptysis | >200 mL/hr in a patient with normal or nearly normal lung function, or >50 mL/hr in a patient with chronic respiratory failure or >2 episodes of moderate hemoptysis (>30 mL) occurring within 24 hours in spite of administration of intravenous vasopressin | 10 |
Lack of consensus regarding this cutoff volume, the known to be unreliable estimation of expectorated volume (e.g., half a cup, a few teaspoons, two tablespoons), and the indiscriminate use of descriptive criteria independent of common nomenclature, as well as other major determinants of morbidity and mortality such as rate of bleeding, the patient’s ability to maintain patent airways, and the extent and severity of cardiopulmonary comorbidities, have led investigators to propose more clinically relevant definitions for what constitutes a major airway bleed.11 These definitions rely on clinical consequences of hemoptysis such as airway obstruction and hemodynamic instability (Table 30-2).12–15 Based on this classification, our patient had massive hemoptysis, in that she required hospitalization for respiratory distress and hypoxemia. It is estimated that it takes approximately 400 mL of blood in the alveolar space to decrease gas exchange significantly, but less is probably required in someone with previously compromised lung function.16 In fact, the cause of death in patients with hemoptysis is usually asphyxiation rather than exsanguination.17 Regardless of how it is defined, massive hemoptysis assessed subjectively or objectively is a major distressing clinical problem for both the patient and the treating physician. In one survey, 86% of responding chest physicians had treated patients with massive hemoptysis during the previous year, and 28% had seen patients die from hemoptysis.18
Table 30-2 Definitions for “Massive” Hemoptysis Based on Clinical Impact
| Defining Term | Clinical Consequence | Reference |
|---|---|---|
| Massive hemoptysis | Transfusion requirement | 12 |
| Massive hemoptysis | Hospitalization | 13 |
| Massive hemoptysis | Endotracheal intubation | 14 |
| Exsanguinating hemoptysis | Aspiration and airway obstruction | 8 |
| Life-threatening hemoptysis | Hypoxemia (PaO2 <60 mm Hg) | 15 |
PaO2, Partial pressure of oxygen in arterial blood.
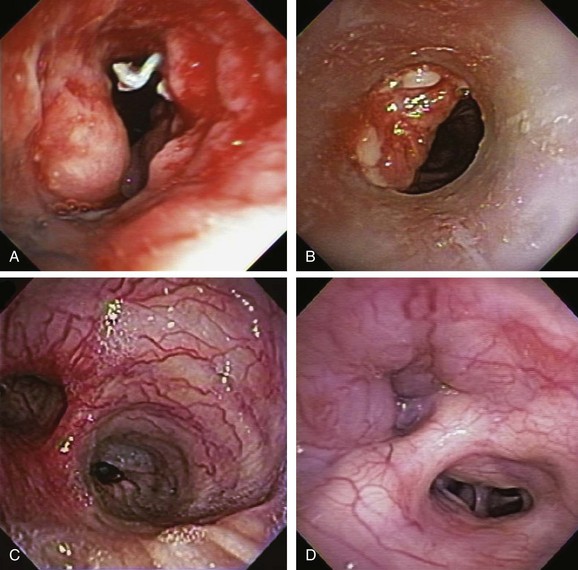
In addition to intrinsic lung disease, increased risks for hemoptysis include a variety of coagulopathic disturbances—congenital, acquired (i.e., uremia), or medication-related (e.g., clopidogrel, bevacizumab). The cause of hemoptysis also tends to be population and region specific. For instance, in Africa and China,* tuberculosis remains a common cause of massive hemoptysis, but this is uncommon in the United States19; in the United States, in fact, the most common causes for hemoptysis are chronic bronchitis, bronchiectasis, and bronchogenic carcinoma, as seen in this patient. These are followed by tuberculosis, fungal infection (e.g., aspergilloma), bacterial pneumonia and abscess, and pulmonary infarction.† The bronchoscopist might encounter hemoptysis caused by granulation tissue overgrowth and erosion of tracheobronchial mucosa from indwelling metal, hybrid, and silicone airway stents (Figure 30-2). Relevant to this patient’s clinical presentation is that hemoptysis occurs in 20% of lung cancer patients at some point during their disease course, with massive episodes developing as the terminal event in 3%.20 Reported mortality rates for massive hemoptysis range from 9% to 38% and depend on rate of bleeding* and cause; the highest mortality rate (38%) was reported in a case series that included a high proportion of patients with advanced carcinoma.†21 In patients with cancer and non–tumor-related hemoptysis (e.g., infection, bronchiectasis), median survival (33 months) was better in comparison with patients who had tumor-related bleeding (2.7 months) following endovascular management.22 These data justify the need to identify the exact cause of hemoptysis in patients in whom cancer has been diagnosed.
Therefore during the initial evaluation of a patient with hemoptysis, a careful bronchoscopic inspection may reveal findings that suggest increased risk for bleeding or findings that could explain the hemoptysis, such as hypervascularization, aberrant vessels, and submucosal arterioles (see Figure 30-2). Pathogenically, the airway inflammation/infiltration associated with carcinoma can cause hypervascularity of bronchial arteries and stimulation of collateral circulation development. Further inflammation or severe coughing causes erosion of these abnormal vascular networks and can result in hemoptysis. Furthermore, in the setting of cancer, angiogenic growth factors may be produced. These promote neovascularization and recruitment of collateral circulation from systemic blood vessels. In this regard, our patient did receive bevacizumab, which was reported to cause hemoptysis (potentially fatal) in 2.3% of patients.*23 Bronchoarterial fistula is of particular concern in patients with tumors invading mediastinal structures. In general, however, a pulmonary arterial origin is responsible for approximately 5% of cases of hemoptysis.24 Large mediastinal vessel rupture secondary to blood vessel wall invasion is very rare but may lead to fatal massive hemoptysis, especially in patients treated with brachytherapy or external beam radiation therapy.25 Multidetector chest computed tomography (MDCT)† with intravenous contrast‡ is very accurate for predicting involvement of nonbronchial systemic arteries in patients with massive bleeding.§ Tumor invasion of the pulmonary artery should not be misread as pulmonary embolism because anticoagulation in this setting could be catastrophic. MDCT is also useful for planning arterial embolization by providing information about bronchial and nonbronchial systemic arteries.24 Although some authors suggest that computed tomography (CT) can replace bronchoscopy as a first-line diagnostic approach in patients with large (<300 mL/24 hr) or massive hemoptysis (>300 mL/24 hr) because of its higher diagnostic yield,7 others find it complementary to bronchoscopy for bleeding site identification.26 MDCT is of considerable diagnostic value for diagnosing underlying disease, and used alone can localize the site of bleeding in 63% to 100% of patients. Combining bronchoscopy and CT increases this yield even further.21 Bronchoscopy is particularly useful in unstable patients with active bleeding who might require endobronchial treatment. Bronchoscopy identifies the site of bleeding 73% to 93% of the time in cases of massive hemoptysis,* but localization rates are significantly lower in cases of mild or moderate hemoptysis.19
Support System
Our patient was divorced; she had two children who were very supportive. They were quite distraught about their mother’s respiratory distress and bleeding and were wondering whether the chemotherapy was helpful for her disease. They had begun inquiries about supportive care measures. They were told that our interpretation of the published evidence was that chemotherapy improves overall survival in patients with advanced non–small cell lung carcinoma (NSCLC) and that those who are fit enough, as their mother had been before this episode (Eastern Cooperative Oncology Group [ECOG] 1), should receive chemotherapy if it is offered. A meta-analysis (2714 patients from 16 randomized controlled studies) of chemotherapy and supportive care versus supportive care alone for NSCLC showed a significant benefit for chemotherapy equivalent to a relative increase in survival of 23%, and an absolute increase in survival of 9% at 12 months. Overall, survival was increased from 20% to 29%, with an absolute increase in median survival of 1.5 months (extending survival from 4.5 months to 6 months).27
Patient Preferences and Expectations
During our conversation, our patient stated that she would undergo any treatments as long as they continued to offer her a good quality of life. She agreed to bronchoscopy with electrocautery to cease the bleeding if possible, but not to intubation and mechanical ventilation, even if respiratory failure was triggered by the bronchoscopy itself. When we asked her why, she said that deep down inside, she believed that intubation was too invasive and was probably “futile.” Sitting at her bedside, surrounded by her family and several physicians-in-training, we asked her what she meant by that. She quoted Star Trek in saying, “resistance is futile,”* and she told us that eventually, death must be accepted. She did not think intubation would prevent her from dying in the very near future, and if anything would probably cause both her and her loved ones to suffer. She insisted that she did not want her daughters to have memories of her on a breathing machine.
According to principles of autonomy and self-determination, patients or their surrogates have the right to refuse procedures regardless of the opinions of their health care providers, so long as they are considered capable of making such decisions, even if the treatment is considered to be life-sustaining. However, the opposite, that is, their right to demand a treatment—especially one that is regarded as “futile” by heath care providers—is much more controversial.28 In this regard, the Texas Advance Directives Act of 1999 provided an extrajudicial conduit for a health care facility to discontinue life-sustaining procedures against patient (or surrogate) wishes.†
Although futility has been described in both qualitative and quantitative terms, today almost all experts would agree that futility cannot be viewed solely as having a single definition but must be considered in context, based on the knowledge, biases, values, and experiences of the individuals involved, and taking into account perceived and accepted goals of care that have been identified for a particular individual. Several succinct definitions have been offered, however, to help clarify the futility issue. One proposed definition for a futile action refers to an action that cannot achieve its goals, no matter how often it is repeated.29 In this sense, however, futility does not refer to something that is impossible to do (as of this writing, systemic chemotherapy is not curative for stage IV lung cancer).
We believe that our patient’s refusal of intubation, regardless of the cause or indication, was likely a reflection of hopelessness and not necessarily a demonstration of her understanding of futility concepts, although her reference to resistance and acceptance can be quite pertinent. Hopelessness is subjective, but futility refers to the objective quality of an action.29 Futility refers to “the expectation of success that is predictably or empirically so unlikely that its exact probability is often incalculable.”29 We respected our patient’s choice, but we took the liberty of explaining to both her and her family that intubation in the setting of hemoptysis causing respiratory distress is not a futile intervention‡ because it could allow us to secure her airway, prevent further damage from ipsilateral or contralateral aspiration, stabilize her respiratory status, and potentially treat her hemoptysis using additional bronchoscopic techniques, or by providing time to perform emergent bronchial artery embolization. We emphasized that these procedures not only would stabilize her condition but could, and in our experience often did, allow extubation and discharge to home.
Procedural Strategies
Indications
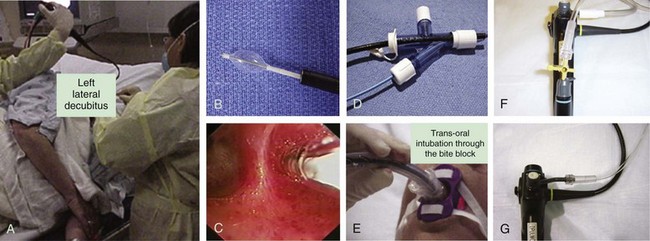
The primary goal of treating an airway bleed is to establish and maintain an open airway to avoid severe hypoxemia and asphyxiation. This is achieved by performing bronchoscopic suction, using large-bore suctioning (e.g., Yankauer suction catheter) of the oral pharynx, and placing the patient in a lateral safety position with the bleeding side down.* This allows face-to-face contact with the patient if the bronchoscopy team is working from the front or side of the patient (Figure 30-3). The lateral position makes it easier for blood and secretions to flow out from the larynx, then into and out of the corner of the mouth. It is easier to suction the mouth and hypopharynx, and the epiglottis does not collapse onto the glottic opening, as it does in the supine position. It also helps avoid excessive collapse of the larynx and prevents upper airway obstruction by the tongue or an edematous upper airway, especially in patients with obstructive sleep apnea (OSA) or in those who have been administered moderate sedation or anxiolytics (these drugs might be beneficial in patients who are fighting for breath, coughing, or becoming increasingly anxious, frightened, and combative because of ongoing hemoptysis).
The second goal of bronchoscopic intervention is to stop the bleeding. This can be attempted by tamponade of the bleeding bronchus using continuous bronchoscopic suction, but in severe, difficult to control cases, unilateral intubation or double-lumen intubation may be necessary.† If intubation is considered, the largest endotracheal tube possible should be inserted. If double-lumen intubation is considered, often times, a flexible bronchoscope with a satisfactorily sized suction channel cannot be inserted, making bronchoscopic aspiration of blood and secretions difficult. Obviously, intubation of the bleeding airway requires practice and additional expertise.
Bleeding can be slowed by using balloons or the rigid bronchoscope (when used) or cotton pledgets for tamponading the airway, or by instilling vasoconstriction agents such as epinephrine (1: 20,000)*30 and cold saline solution.†31 Lavage with normal saline at 4° C in 50 mL aliquots (average volume, 500 mL; range, 300 to 750 mL) stopped the bleeding in 23 patients with massive hemoptysis (>600 mL/24 hr).‡31 Endobronchial instillation of tranexamic acid (500 to 1000 mg) was reported to immediately (within seconds) control airway bleeding (estimated at 600 to 750 mL), which did not respond to initial conservative therapy (cold saline, epinephrine).32 To further enhance clot formation, the lateral decubitus position is probably useful through its gravity effect. Topical hemostatic tamponade using oxidized regenerated cellulose mesh, a sterile kitted fabric, has also been tried successfully in 56 of 57 (98%) patients with “life-threatening” hemoptysis.§15 If a tamponade balloon or a Fogarty catheter (these can be used in segmental airways but usually are too small to occlude a lobar bronchus), a pulmonary artery balloon catheter (these Swan-Ganz catheters also are usually too small), or an endobronchial blocker (see Figure 30-3) is inserted into a bleeding segmental, lobar, or mainstem bronchus, its position should be verified by flexible bronchoscopy and chest radiograph because these items have a tendency to easily migrate out of the target airway. These devices can remain in place for several days if necessary. As has been mentioned, when these devices are considered, the bronchoscopist and assistants should first verify that the balloon diameter will be sufficient to completely occlude the target segmental, lobar, or mainstem bronchial airway, and that the balloon catheter fits through the working channel of the bronchoscope. The Cook (Arndt) bronchial blocker, for instance, does not fit through the working channel of a flexible bronchoscope, but if necessary, it can be placed using a dedicated adapter (see Figure 30-3) by inserting the catheter alongside (![]() Video VI.30.1) or through (
Video VI.30.1) or through (![]() Video VI.30.2) a large endotracheal tube (see videos on ExpertConsult.com). In our experience, after a clot forms, it is wise to not immediately remove it, especially if bleeding has stopped. Inspection bronchoscopy, with or without clot removal, can be performed at a later time.
Video VI.30.2) a large endotracheal tube (see videos on ExpertConsult.com). In our experience, after a clot forms, it is wise to not immediately remove it, especially if bleeding has stopped. Inspection bronchoscopy, with or without clot removal, can be performed at a later time.
The third goal in managing hemoptysis is to prevent and treat respiratory, cardiac, and hemodynamic complications resulting from severe hypoxemia and hypercarbia. During flexible bronchoscopy, overuse of anxiolytics and narcotics should be avoided, if possible, when patients have significant hemoptysis, because these drugs will suppress cough, reduce minute ventilation, and thus worsen gas exchange. In addition, reduced consciousness and cough suppression may contribute to aspiration of upper airway secretions and aspiration pneumonia. Sedation or anxiolysis might warrant intubation even after bleeding is controlled. If intubation is warranted, a large single-lumen endotracheal tube usually can be inserted over the bronchoscope,* preferably through the mouth with the patient wearing a bite block. Haste makes waste, and we have seen on more than one occasion a combative or fearful patient spit out a poorly secured bite block only to bite down on the fragile flexible bronchoscope. Endotracheal tubes are not long enough to extend unilaterally into a mainstem bronchus if placed through the nares. Therefore selective unilateral bronchial intubation is possible only if the oral route is used† (see Figure 30-3). We do not advise selectively intubating the right main bronchus in a case of bleeding originating from the left lung, because this procedure usually occludes the right upper lobe bronchus, allowing ventilation to the right middle lobe and lower lobes only, and potentially further compromising gas exchange. Instead, we suggest that tracheal intubation be performed for left-sided bleeds, followed by insertion of a balloon catheter through or adjacent to the endotracheal tube, with subsequent introduction into the left main bronchus under bronchoscopic visualization.
Expected Results
Most mild to moderate mucosal bleeding can be controlled by using electrocautery via flexible or rigid bronchoscopy when lesions are visible. Several studies have showed an immediate response in controlling hemoptysis in 70% to 100% of patients.34,35 The main disadvantage of using electrocautery for managing hemoptysis is loss of effectiveness due to diffusion of the current across a large surface area and wet tissues. The procedure may be more time-consuming than with laser because it is a contact mode therapy with more superficial tissue coagulation (2 to 3 mm for electrocautery vs. 5 to 10 mm penetration depth for neodymium-doped yttrium aluminum garnet [Nd:YAG] laser). Thus more debridement and probe cleaning are required. With any electrosurgery, there is a need for smoke evacuation. This requires intermittent suctioning, which may decrease tidal volumes and worsen hypoxemia in an already frail patient undergoing flexible bronchoscopy. Airway perforation, endobronchial fires and bronchoscope damage, bronchomalacia and stenosis, and pacemaker and automated implantable cardioverter/defibrillator (AICD) dysfunction are rarely reported complications of electrosurgery.36,37 During the electrocautery application, the bronchoscopist should be aware that cough induced when the probe touches the tumor can promote additional bleeding, and that blood can easily spread throughout the tracheobronchial tree, obscuring the bleeding site and potentially leading to severe hypoxemia and asphyxiation.
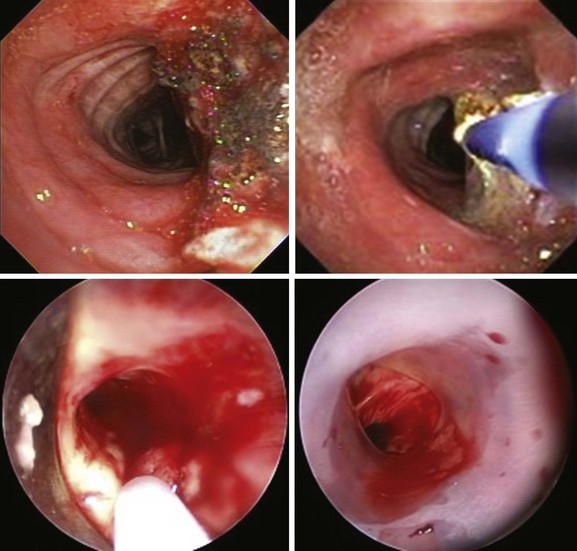
Argon plasma coagulation (APC) as a noncontact electrocautery mode could be chosen instead of contact probe electrocautery because it provides easy access to lesions located laterally or around anatomic corners (Figure 30-4).38 In addition, APC allows homogeneous tissue desiccation (see video on ExpertConsult.com) (![]() Video VI.30.3) because it seeks areas with higher water content and less electrical impedance. In a retrospective study, 31 patients with hemoptysis and 25 patients with both airway obstruction and hemoptysis were treated by endobronchial APC therapy. Bleeding was quantified as severe (>200 mL/24 hr) in 6 patients, moderate (50 to 200 mL/24 hr) in 23 patients, and mild (<50 mL/24 hr) but persistent for more than a week in 27 patients. Airway hemorrhage stopped immediately after the procedure in all patients with an endoluminal tumor responsible for the bleeding.38
Video VI.30.3) because it seeks areas with higher water content and less electrical impedance. In a retrospective study, 31 patients with hemoptysis and 25 patients with both airway obstruction and hemoptysis were treated by endobronchial APC therapy. Bleeding was quantified as severe (>200 mL/24 hr) in 6 patients, moderate (50 to 200 mL/24 hr) in 23 patients, and mild (<50 mL/24 hr) but persistent for more than a week in 27 patients. Airway hemorrhage stopped immediately after the procedure in all patients with an endoluminal tumor responsible for the bleeding.38
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree