Chapter 75
Hemodialysis Access
Complex
David L. Cull
Based on a chapter in the seventh editon by Brendon M. Quinn and David L. Cull
Significant improvements have been made in quality of care and life expectancy for patients on dialysis over the past decade. Consequently, it is not uncommon for the surgeon to be confronted with patients who have “outlived” their arteriovenous (AV) access options in the upper extremities. In one study, nearly 7% of access placements were located at a site other than the upper extremity.1 For many of these patients, quality of life and long-term survival depend primarily on the surgeon’s ability to provide a functional and durable AV access. To meet this challenge, the access surgeon must have a number of complex vascular access procedures in his or her surgical armamentarium and must be aware of the advantages and disadvantages of each.
The staggering morbidity and financial burden associated with vascular access have prompted efforts, such as the National Kidney Foundation’s Kidney Dialysis Outcomes Quality Initiative (KDOQI), to use principles of evidence-based medicine to determine the outcome of access procedures and ultimately to standardize AV access management.2 These efforts have resulted in the development of clinical practice guidelines for vascular access that include an order of preference for AV access procedures and emphasize autogenous AV access creation. However, there is a paucity of evidence-based literature related to the outcome of “complex” access procedures that become necessary when options in the upper extremity are exhausted. The literature reporting the outcome of complex AV access procedures in the lower extremity and chest wall consists of only a few small series or case reports. Consequently, the KDOQI clinical practice guidelines are unhelpful when it comes to recommendations for providing access in patients in whom AV access placement in the upper extremity is not possible.
What is apparent from the available literature and our own anecdotal experience is that these complex access procedures are associated with a higher complication rate compared with AV access procedures of the upper extremity, and the management of these complications is generally more challenging. Although one might be tempted to avoid complex vascular access procedures by simply placing a tunneled dialysis catheter, the significant complications associated with chronic dialysis via a catheter are well established.3–5 The KDOQI guidelines for vascular access recommend use of a dialysis catheter only as a bridge to AV access placement or in patients with an extremely limited life expectancy.2 In one series reporting the outcome of prosthetic femoral-femoral AV accesses, the median survival for patients undergoing the procedure was 24 months.6 Therefore despite the challenges of complex AV access placement and the associated complications, their placement is usually justified and preferable to a tunneled dialysis catheter.
General Principles
When facing a complex access situation, the surgeon must obtain a complete access history and delineate the cause of prior failures. Valuable information is often gained from previous operative notes, nephrologists, and dialysis nurses. A careful investigation of the vascular anatomy is important to identify arterial or venous pathology that may affect access outcome. Although noninvasive vascular testing is useful, it may provide insufficient anatomic information for patients who have had multiple access procedures; therefore, contrast angiography (either CT or catheter-based) is often required. Because upper extremity access can cause venous stenosis or occlusion at sites remote from the access, and because many of these patients have had previous central venous catheters, venography is often necessary to exclude central venous stenosis or occlusion. Only after a complete understanding of the history and anatomy can the surgeon consider all access options and create a long-term access strategy for the patient.
Avoiding Complex Access
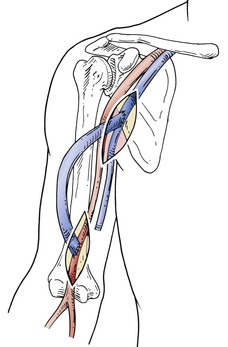
As noted earlier, the complication rate of AV access procedures of the lower extremity, chest wall, and other “exotic” access sites is high, and these complications are difficult to manage. Therefore one should ensure that alternatives in the upper extremity do not exist before resorting to these locations. Even though a patient has had multiple failed AV accesses in the extremity, a venogram may reveal an alternative vein, such as a paired brachial vein or a patent cephalic vein in the deltopectoral groove that can provide venous outflow for an additional access procedure in the extremity. Such options should be used before moving to sites outside the upper extremity (Fig. 75-1). If a central venous stenosis is present and the vessels in the upper extremity appear adequate for AV access placement, the surgeon should consider angioplasty and stenting of the central vein stenosis and then placement of an upper extremity access, rather than proceeding with a complex access elsewhere. Although the primary patency of percutaneous central vein angioplasty is only 29% at 12 months, remedial angioplasty procedures either alone or with a stent are generally easy to perform and can extend the 12-month patency to more than 70% (Fig. 75-2).7
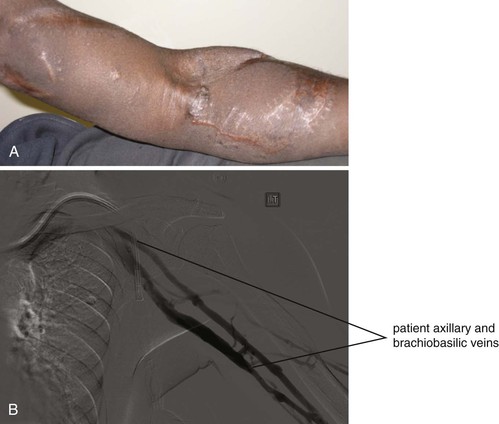
Figure 75-1 A, Patient with failed prosthetic AV accesses in the left forearm and upper arm is referred for the establishment of a new access. B, A venogram was obtained to determine whether any alternative to a “complex” access was available in the left upper extremity. Despite the two previous upper extremity AV access procedures, the brachiobasilic, axillary, and central veins were widely patent. A successful brachial-basilic transposition was performed.
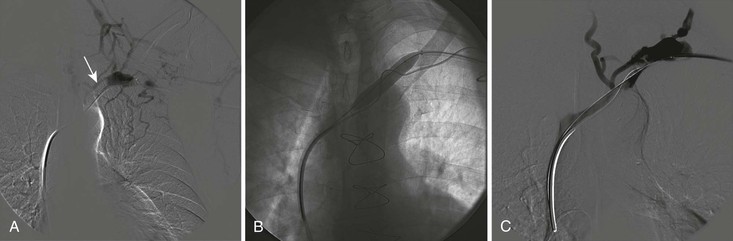
Figure 75-2 A, Venography of the left upper extremity in a patient with an autogenous brachial-cephalic AV access and symptoms of venous hypertension demonstrates chronic occlusion of the subclavian vein (arrow). B, The subclavian vein occlusion was crossed with a wire, and angioplasty was successfully performed with a high-pressure balloon. C, Completion venography demonstrating patency of the central veins.
Complex Access Site Selection
Although algorithms have been developed that define a general order of preference for access placement in the upper extremity, the development of a similar algorithm for complex access placement is problematic, given the dearth of evidence-based literature related to these procedures. It is possible, however, to provide broad recommendations regarding the clinical situations in which a particular complex AV access procedure is most helpful and those in which it should be avoided. These recommendations are outlined in Table 75-1.
Autogenous AV Access
Over the past decade, major emphasis has been placed on the use of autogenous AV accesses in preference to prosthetic accesses because of their higher primary patency rates and lower infection rates. Several techniques have been described that use the saphenous and femoral veins to create autogenous AV accesses in the upper and lower extremities. These veins can be completely mobilized and disconnected both proximally and distally to create an access at a site remote from their origin (translocation). Alternatively, the distal portion of the vein can be mobilized and tunneled superficially, leaving the central portion of the vein connected to its normal anatomic position (transposition).
Given the high infection rate associated with AV access procedures of the lower extremity, use of the saphenous vein or femoral vein translocation or transposition procedures in the thigh is theoretically appealing. However, these operations have been associated with wound complications related to vein harvest and access-related ischemia or steal syndrome; therefore their role in prosthetic AV access procedures remains undefined, and they are not included in the KDOQI clinical practice guidelines.2
Translocation Procedures
Saphenous Vein–to–Forearm Translocation
Three studies have been published evaluating saphenous vein–to–forearm translocation for hemodialysis access. In 1980, May et al reported the long-term results of a series of 71 saphenous vein–to–forearm loop translocations.8 The secondary patency rates were 77% at 1 year and 66% at 2 years. Infection of the access occurred in 4% of cases. In Bhandari et al’s series of 29 saphenous vein translocations, the saphenous vein was placed in a straight configuration between the radial artery and an antecubital vein.9 The secondary patency rate was 89% at 1 year, and no infections occurred. Smith et al recently reported a series of 24 patients who underwent saphenous vein–to–forearm loop translocation.10 The primary and secondary patencies at 12 months were 41% and 50%, respectively. Since patency is not clearly defined in any of the saphenous vein–to–forearm translocation reports, it is unclear whether the patency results cited in those studies refers to functional patency. Therefore the actual patency for saphenous vein–to–forearm translocation may be less than that reported by these series.
Femoropopliteal Vein–to–Arm Translocation
Results.
Huber et al reported a series of 30 patients who underwent translocated femoral vein–to–upper arm brachial-axillary access.11 They reported primary and secondary patency rates of 79% and 100% at 12 months and 67% and 100% at 18 months. Clinically significant access-related ischemia of the upper extremity occurred in 27% of patients in that series. Each patient underwent a distal revascularization–interval ligation (DRIL) procedure to treat severe ischemia and thereby salvage the access. Huber’s group stressed the importance of using the contralateral rather than the ipsilateral saphenous vein for the DRIL procedure to reduce the risk of limb swelling and compartment syndrome after the initial femoral vein harvest. Two patients (7%) developed compartment syndrome necessitating fasciotomy. Wound hematomas developed at the vein harvest site in 23% of patients and in the arm in 17% of patients. Although this procedure has potential advantages because it is an autogenous access, those advantages must be balanced against a high incidence of wound complications and access-related upper extremity ischemia.
The autogenous brachial-axillary femoropopliteal vein translocation is an alternative to thigh access for patients with poor superficial upper extremity veins who are not candidates for a traditional upper extremity autogenous access. This access may also be indicated for patients with multiple failed upper extremity prosthetic AV accesses owing to infection or unexplained thrombosis.
Technique.
The preoperative evaluation should include an assessment of the arterial and venous anatomy of both the lower and upper extremities. Before harvesting the femoral vein, the surgeon must ensure that the patient has adequate arterial circulation to heal wounds from the vein harvest. Duplex ultrasonography of the lower extremity is necessary to confirm that the femoral vein is patent and has an adequate diameter (>6 mm).
A longitudinal skin incision is made over the brachial artery proximal to the antecubital fossa, and a 3-cm segment of the artery is exposed. The axillary vein is exposed through a single longitudinal incision in the axilla and proximal upper arm. To expose the femoral vein, an incision is made in the groin and extended distally along the medial border of the sartorius muscle. The sartorius muscle is retracted laterally in the proximal thigh and medially in the distal thigh to facilitate exposure of the femoral and popliteal vein. The vein is mobilized from the mid–popliteal fossa to the common femoral vein. Tributaries off the vein are ligated and divided. The femoral vein is harvested flush with the profunda vein. It is important to preserve the profunda vein to minimize the occurrence of compartment syndrome. A tunnel is created between the brachial artery and axillary vein over the ventral aspect of the upper arm. The vein is reversed and passed from the brachial artery to the axillary vein. An end-to-side anastomosis is performed between the femoral vein and the axillary artery with a 5-0 or 6-0 polypropylene suture (Fig. 75-3). A closed suction drain is placed in the thigh wound, and the incisions are closed in layers. Because of the significant size mismatch between the femoral vein and the brachial artery, there may not be enough turbulence in the vein to feel a thrill initially.
Transposition Procedures
Brachial Vein Transposition
Results.
In 2004, Bazan and Schanzer reported two cases of autogenous brachial vein transposition in patients with inadequate superficial upper extremity veins for autogenous AV access creation.12 No manifestations of venous hypertension occurred in either patient. After 1 year, both accesses were functional. Subsequently, Elwakeel et al reported a series of 21 patients who underwent brachial vein transposition.13 In contrast to Bazan and Schanzer’s technique, they used a two-stage procedure. During the first stage, the brachial artery–brachial vein anastomosis was performed at the antecubital fossa. If the brachial vein at the antecubital fossa measured less than 3 mm, the procedure was abandoned and a prosthetic graft was placed. Approximately 1 month after the first-stage procedure, the patients underwent a second step “superficialization” procedure. This procedure involved the mobilization of the arterialized brachial vein from the antecubital fossa to the axilla. The mobilized access was then transposed to a subcutaneous pocket lateral to the incision. All but one patient in this series had undergone previous upper extremity AV access procedures. The functional patency rate was 76% at 1 year and 55% at 2 years. Three accesses failed to mature. Although four patients experienced arm edema postoperatively, it resolved spontaneously in all cases. Angle and Chandra reported a series of 20 patients who underwent a two-stage procedure similar to that reported by Elwakeel, except that all the procedures were first-time accesses.14 Of the 20 AV accesses, 19 (95%) matured and were functional; patency rates were not reported. One patient with pacemaker wires in the subclavian vein ipsilateral to the access developed venous hypertension secondary to central vein stenosis. Casey et al reported a series of 17 brachial vein transpositions.15 Only patients with brachial vein diameters greater than 4 mm on preoperative vein mapping were considered candidates for the procedure. All were performed as single-stage procedures. Fifty-three percent of the brachial vein transpositions in this series failed to mature. The functional primary and secondary patency rates were 40% at 12 months. In 2009, Jennings reported outcomes of a series of 58 brachial vein transpositions.16 The majority were performed as two-stage procedures. Single-stage procedures were performed only in cases in which the brachial vein initially measured more than 6 mm in diameter on preoperative vein mapping. The primary and secondary patency rates were 52% and 92% at 12 months.
In addition to the studies cited previously, several other brachial vein transposition series have recently been reported. However, those reports failed to use standard methods for reporting vascular access outcome and thus were not included in this review. None of the published series on brachial vein transposition report follow-up long enough to obtain reliable patency estimates beyond 1 year.
Technique.
Upper extremity vein mapping is performed to determine the diameter and quality of the brachial, cephalic, and basilic veins. Brachial vein transposition should be considered as an option for vascular access only if the brachial vein diameter is greater than 2.5 mm and in cases in which vein quality precludes the creation of a brachial-cephalic autogenous access or basilic vein transposition. Most reports recommend that brachial vein transposition be performed as a two-stage procedure whereby the AV anastomosis is created at the first stage, followed 6 weeks later by vein transposition. One-stage procedures are considered only in cases in which the brachial vein exceeds 4 mm in diameter. The first stage (creation of the AV anastomosis) is performed using local anesthesia. For the second stage (mobilization and transposition of the vein), local sedation can be used in selected cases; however, the preferred anesthetic technique is either a regional (interscalene) block with sedation or general anesthesia. The first stage of the procedure involves making a longitudinal incision over the brachial pulse in the antecubital fossa in order to expose both the brachial artery and vein. When multiple brachial veins are present, the largest vein is used for the AV anastomosis. Use of the proximal radial artery, rather than the brachial artery, as the site for the AV anastomosis may reduce the risk of access-related limb ischemia. An end-to-side vein-to-artery anastomosis is performed using a running 6-0 polypropylene suture. The second stage is performed 4 to 6 weeks after the first to allow the vein wall to arterialize, thereby facilitating its mobilization. A longitudinal incision is made over the course of the brachial artery and vein in the distal upper arm. This incision is extended proximally several centimeters at a time while sequentially freeing up the anterior surface of the vein in order to expose the entire brachial vein length from the antecubital fossa to the axilla.
Mobilization of the vein can be challenging and thus requires considerable patience. The medial antebrachial cutaneous and median nerves are adjacent to the vein and must be protected from injury. Also, because the vein may be adherent to the adjacent brachial artery, it can easily be torn during mobilization. Venous tributaries are individually ligated with 4-0 silk sutures. Short, broad tributaries are oversewn with 7-0 polypropylene. After mobilization, the anterior surface of the vein is marked to prevent twisting during transposition. A number of techniques for transposing the vein have been described. If the length of mobilized vein is adequate, the preferred technique is to tunnel it through a separate subcutaneous tunnel over the ventral aspect of the upper arm. For this, a tunneling device is passed lateral to the upper arm incision from antecubital fossa to axilla. The vein is divided near the AV anastomosis and passed through this subcutaneous tunnel, taking care to prevent twisting or kinking. An end-to-end anastomosis of the vein is performed using a 6-0 polypropylene. If the vein is too short to be transposed through a separate subcutaneous tunnel, it can be simply elevated by closing the fascia and subcutaneous tissue of the wound beneath it with interrupted 3-0 absorbable suture. A running 4-0 absorbable suture is used to close the skin over the vein containing the access. Postoperatively, dialysis personnel are instructed to cannulate the AV access directly through the overlying scar. Alternatively, a subcutaneous flap (approximately 3 mm below the skin) can be developed anterior to the skin incision. Placement of the access in this pocket positions it away from the skin incision. The access can be cannulated once the skin incision has completely healed, usually in about 3 weeks after the second-stage procedure.
Saphenous Vein Transposition
Results.
The common femoral artery–saphenous vein loop transposition was first described in a single patient by May et al in 1969.17 Pierre-Paul et al recently reported a series of seven patients who underwent the procedure.18 Two of the seven accesses (29%) did not achieve functional maturation. Local wound complications from the vein harvest incision occurred in more than half the patients. Secondary interventions to maintain access patency were necessary in all cases (mean of three angioplasties per access). The mean time to secondary failure was 16 months. Gorski et al reported a series of five saphenous vein transpositions in patients with acquired immunodeficiency syndrome.19 The primary and secondary patency rates were 80% at 1 year. The only failure occurred as a result of bleeding from early cannulation of the access. In 2002, Illig et al reported a series of four patients who underwent the procedure.20 In an effort to minimize the wound complications reported in other series, an endoscopic technique was used to harvest the greater saphenous vein. One access failed early; functional patency in the remaining accesses was achieved for 6 months, 12 months, and 13 months. No major wound complications or infections occurred.
Based on the limited number of series reporting the outcome of saphenous vein translocation, the following conclusions can be made: (1) The use of skip incision or endoscopic techniques to harvest the saphenous vein may decrease the wound complications associated with this procedure. (2) Because the great saphenous vein does not readily dilatate after access creation, only veins greater than 3 mm in diameter should be used, and the vein must be tunneled just beneath the dermis to allow reliable cannulation of the access. (3) Cannulation of the access must be delayed at least 6 weeks postoperatively to prevent puncture site bleeding and hematoma. (4) The procedure may not be practical for patients who are morbidly obese or those with a large, redundant pannus, because access cannulation may require the patient to lie in the supine position and retract the pannus to expose the access.
Technique.
The saphenous vein is exposed and mobilized from the saphenofemoral junction to the knee. The saphenous vein is transected distally at the knee and harvested, leaving the saphenofemoral junction intact. The proximal superficial femoral artery is exposed. A subcutaneous tunnel is developed next to the dermis, in the anterior aspect of the thigh, through two separate skin incisions. The saphenous vein is brought through the tunnel in a loop configuration and anastomosed end to side to the proximal superficial femoral artery. Alternatively, mobilization of the saphenous vein can be accomplished using skip incisions over the vein or an endoscopic vein harvest technique.
Femoral Vein Transposition
Results.
The largest experience with autogenous femoral artery–femoral vein transpositions was reported by Gradman et al in two separate series.21,22 The initial series reported the outcome for 25 patients who underwent the procedure. Ten of the accesses were narrowed or “banded” (i.e., flow limited) at the time of the initial procedure to prevent postoperative access-related limb ischemia. Even with banding, limb ischemia necessitating additional surgery was common, occurring in 32% of patients. The primary and secondary patency rates at 12 months were 78% and 87%, respectively. The ankle-brachial index decreased an average of 0.21. Major wound complications occurred in 28% of cases. One patient developed compartment syndrome requiring fasciotomy and ultimately required an above-knee amputation.21
In an effort to decrease the incidence of access-related limb ischemia associated with this procedure, Gradman modified the technique and patient selection criteria, reporting the outcome in a separate series of 22 patients.22 To prevent access-related limb ischemia, the size of the arterial anastomosis was limited to 4.5 to 5 mm. This was accomplished by inserting a 5-mm mandrel in the beveled vein and closing the excess vein with suture. The tapered vein was then anastomosed end to side to the distal superficial femoral artery. Patients with an ankle-brachial index less than 0.85 or absent pedal pulses were excluded. Prophylactic fasciotomies were performed in patients with weak or absent pedal pulses after access creation. The secondary patency rate in this second series was 94% at 2 years. With the modification in technique and patient selection criteria, the occurrence of limb ischemia necessitating revascularization was reduced from 32% to 0%. Bourquelot recently reported outcomes from a series of 72 femoral vein transpositions.23 Patients with diabetes mellitus or peripheral artery disease were not considered candidates for the procedure. The primary patency was 91% at 1 year. The secondary patency was 84% at 2 years. Major complications requiring access ligation was required in 13 (18%) of patients. These complications included five cases of distal limb ischemia, two cases of venous hypertension, two cases of bleeding, and one case of high-output heart failure. In a case report, Jackson reported flow rates for femoral vein transposition exceeding 2000 mL/min.24 Therefore caution is necessary when creating this access in patients with congestive heart failure.
Technique.
A preoperative assessment of the lower extremity arterial and venous anatomy with duplex ultrasonography is necessary. Complication rates for this operation may be reduced through careful patient selection and modification of the procedure for patients who are at high risk for complications.
The femoral vein is exposed and mobilized as described previously. In obese patients it is often necessary to mobilize the vein to the popliteal vein at the knee to gain adequate length. The profunda femoral vein is an important collateral vessel; therefore it is important to preserve its continuity to prevent venous hypertension and compartment syndrome. The femoral vein is ligated distally and transected. The vein is brought through a subcutaneous tunnel lateral to the vein harvest incision to the superficial femoral artery in the distal thigh. The distal end of the femoral vein should be tapered to 4.5 to 5 mm as described by Gradman and outlined earlier (Fig. 75-4A). An end-to-side anastomosis is performed to the distal superficial femoral artery with a 6-0 polypropylene suture.
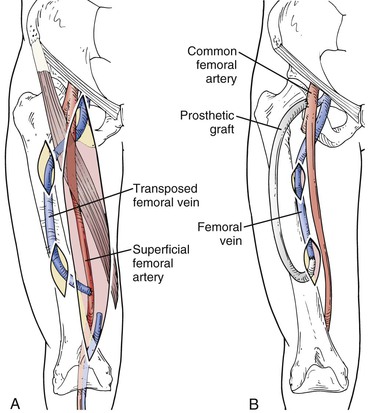
Figure 75-4 A, Femoral vein transposition procedure. To expose the femoral vein, see the Femoropopliteal Vein–to–Arm Translocation technique section. After the femoral vein is exposed, it is transected in the popliteal fossa and mobilized to the common femoral vein, preserving the profunda vein. The femoral vein is passed posterior to the superficial femoral artery through a subcutaneous tunnel on the anterior thigh and anastomosed to the distal superficial femoral artery. B, Composite femoral vein transposition and prosthetic access procedure. The 4- to 7-mm polytetrafluoroethylene graft is tunneled deeply to prevent needle cannulation of the prosthetic access and to reduce infection. This technique is used in patients at high risk for access-related limb ischemia or in obese patients with inadequate vein length for superficialization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree