Chapter 73
Hemodialysis Access
General Considerations
Thomas S. Huber
Based on a chapter in the seventh edition by Robyn A. Macsata and Anton N. Sidawy
End-stage renal disease (ESRD) is a huge public health problem with significant morbidity, mortality, and cost. The national initiatives and guidelines have helped define the standard of care for vascular access and have emphasized the role of autogenous arteriovenous (AV) hemodialysis access. Although a mature autogenous access likely represents the optimal access choice, the real goal is a functional access with minimal associated complications. The construction and maintenance of hemodialysis access represent a significant component of many vascular practices. Despite their relatively minor magnitude, permanent access procedures are associated with significant perioperative morbidity and mortality, underscoring the patients’ underlying comorbidities. Maintaining effective hemodialysis access is a lifelong challenge that requires a long-term plan and committed providers.
End-Stage Renal Disease
Epidemiology
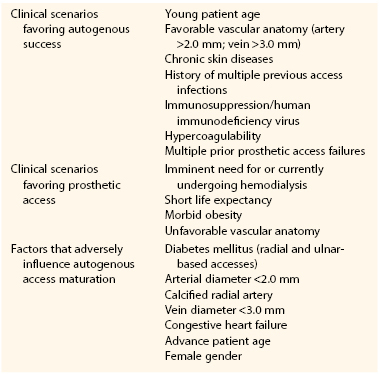
ESRD, and the maintenance of hemodialysis access, is a tremendous public health problem that has reached almost epidemic proportions in the United States. In 2010, there were 594,374 prevalent (prevalence = patient count at a single point in time) and 116,946 incident (incidence = new patients over a time interval) cases of ESRD.1 Notably, this represents more than tenfold increase from 1980, and it has been estimated that this trend will continue, with estimated prevalent and incident counts of 784,613 and 150,772, respectively by 2020 (Fig. 73-1).2 The majority of patients with ESRD are on hemodialysis (hemodialysis 65%, transplantation 30%, peritoneal dialysis 5%).1 Approximately 17,000 kidney transplants were performed in 2010, but the number of patients awaiting transplant has continued to increase and has outstripped the number of available organs. Indeed, more than 87,000 patients in the United States were awaiting a kidney transplant in 2010, with the median time on the transplant list of 1.71 years.1 This gap between the supply and demand of kidney transplants is expected to continue to widen. The majority of patients initiated dialysis in 2010 (i.e., first dialysis session) using a catheter as the sole access (catheter 81%, fistula 16%, graft 3%).1 However, claims data suggest that there was some reduction in catheter usage within the first 4 months (catheter 53%, fistula 17%, graft 3%, unknown 27%).1
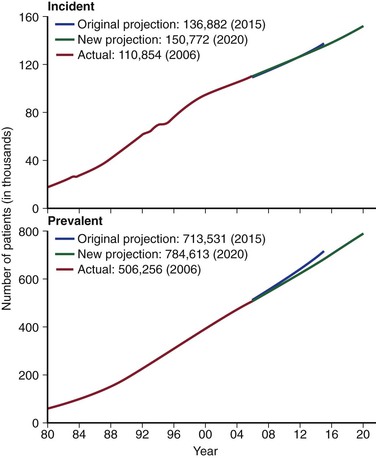
Figure 73-1 The projected incidence and prevalence for patients with ESRD for years 1980 to 2020 is shown. Note the continued growth of both patient cohorts. (From U.S. Renal Data System: USRDS 2008 annual data report: atlas of end-stage renal disease in the United States, vol 2, Bethesda, MD, 2008, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Available at http://www.usrds.org/atlas08.aspx.)
Morbidity and Mortality
The mortality and morbidity associated with ESRD and maintaining access are significant. The unadjusted 1-year mortality rate for hemodialysis patients in the United States was 22% as reported from the Dialysis Outcomes and Practice Patterns Study (DOPPS) with the incident mortality rate highest within the first 120 days after initiation.3,4 The majority of the early deaths are related to cardiovascular and infectious causes, with the presence of a central venous catheter and hypoalbuminemia identified as predictors.1,5 The mean life expectancy for all patients with ESRD in the United States is 5.8 years and, predictably, varies by age, gender, race, and renal replacement therapy.2 Notably, the life expectancies for patients 50 to 54 years and 80 to 84 years of age are 6.3 and 2.3 years, respectively.2 Patients whose initial ESRD treatment is a transplant are 2.3 times more likely to survive 5 years (survival probability: transplant 0.74, hemodialysis 0.33, peritoneal dialysis 0.33).1 Patients undergoing dialysis (both hemodialysis and peritoneal) are admitted to the hospital approximately twice per year whereas those with transplants are admitted once.1 These admissions account for a mean 12.1 hospital inpatient days per year for the dialysis patients and 5.5 days for those with transplants.1 The corresponding principal admission diagnoses are dialysis-related infection > bacteremia/septicemia > pneumonia > cellulitis.1 Patients on hemodialysis are more likely to be admitted with each of these diagnoses than those on peritoneal dialysis or with a transplant.1 The annual event (i.e., removal, replacement with catheter, replacement with internal device) and complication (i.e., infection, sepsis) rates for hemodialysis catheters exceed those for autogenous and prosthetic AV accesses, with reported values of 0.7 and 2.1 episodes/year, respectively.1 The event (i.e., replacement with prosthetic access, replacement with autogenous access, replacement with catheter, revision, removal) and complication (i.e., infection, sepsis, declot, angioplasty) rates are lowest for patients with autogenous accesses (event rate 0.25 vs. 0.13, complication rate 0.77 vs. 0.44) although the values are comparable to rates for those with prosthetic accesses.1
Cost
The Medicare costs associated with ESRD are staggering. Although ESRD accounts for only 1.3% of the general Medicare population, it accounted for 7.5% ($25.7 billion) of the general Medicare costs in 2010.1 Notably, Medicare is the primary payer for patients with ESRD in the United States and is responsible for more than 80% of the patients.1 The Medicare expenditure per patient with ESRD in 2006 was greatest for patients on hemodialysis (hemodialysis $71,889, peritoneal dialysis $53,327, transplant $24,951).2 Among those patients undergoing hemodialysis, the expenditure (patient per year) was greatest for those with catheters (catheters $77,093, grafts $71,616, fistulae $53,470).2
Renal Replacement Therapy
Vascular surgeons are predominantly involved in the care of patients with chronic kidney disease (CKD) and ESRD in terms of establishing and maintaining access for hemodialysis. However, it is important to emphasize that there are other alternatives for renal replacement therapy. Kidney transplantation is likely the best option for most patients, provided that they are suitable candidates. Indeed, in almost every single outcome measure (e.g., life expectancy, quality of life, inpatient admission), kidney transplantation is superior to hemodialysis and peritoneal dialysis. Peritoneal dialysis is also an attractive alternative to hemodialysis and has many advantages. It allows a more flexible lifestyle but requires a committed patient and/or family. Although the focus of this chapter and section is hemodialysis access, these other alternatives should be addressed with the patient and the nephrologist, particularly those patients with limited (or exhausted) AV access options.
Guidelines and National Initiatives
Hemodialysis access care over the past 2 decades has been largely shaped by the national guidelines and initiatives.
National Kidney Foundation
Foremost among the initiatives and guidelines is the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Recommendations for Vascular Access.6 The recommendations were originally published in 1997 as the Dialysis Outcome Quality Initiative (DOQI) and then updated in 2000 and 2006 as KDOQI to reflect the expanded emphasis on quality improvement for kidney disease. The KDOQI recommendations were developed through the use of an evidence-based approach encompassing an exhaustive, critical review of the literature by a panel of experts. The stated goals included increasing the number of autogenous AV accesses and detecting access dysfunction prior to thrombosis. The KDOQI recommendations comprised a series of individual guidelines (Box 73-1) that address the various components of access care, including the selection of a specific access configuration, access placement goals, and the management of complications. These guidelines emphasize autogenous AV access, as might be suspected by the overarching goals, with the generic recommendations that autogenous access should be used before prosthetic access and that dialysis catheters should be avoided. The expert panel defined the autogenous radial-cephalic, brachial-cephalic, and brachial-basilic AV accesses as “preferred” and stated that the prosthetic AV access was “acceptable.” The latest version of the KDOQI guidelines established a 65% goal for autogenous access among prevalent dialysis patients with a catheter rate of less than 10% in the absence of a maturing autogenous access. The expert panel stated that the patency rate for autogenous access should be more than 3 years by life-table analysis with thrombosis and infectious rates of less than 0.25 episodes/patient-year at risk and less than 1% over the lifetime of the access, respectively. Similarly, the patency rates for prosthetic accesses should be more than 2 years with a thrombosis rate of less than 0.5 episodes/patient-year and an infection rate of less than 10% over the lifetime of the access.
Centers for Medicare and Medicaid Services
The National Vascular Access Improvement Initiative, or Fistula First Breakthrough Initiative, was a Centers for Medicare and Medicaid Services (CMS) project, initially spanning 2003 through 2006, that was designed to increase the proportion of hemodialysis patients using autogenous AV access as the primary mode of dialysis.7 The stated mission of the Fistula First was to maximize autogenous access in all suitable patients, minimize catheter use, and avoid all types of complications. These laudable goals were implemented through the ESRD networks using a series “change concepts” (Box 73-2). Notably, there were 11 initial “change concepts,” with two others added later. A variety of different tools was used to implement these concepts, including multimedia resources and provider-specific education and feedback. The initial autogenous AV access goals of the Fistula First Initiative were identical to those of the original DOQI (incident 50%, prevalent 4), but were raised to 66% prevalence rate by 2009. A later report suggests that these efforts have been largely successful across the United States as reflected by the trend in autogenous and prosthetic accesses (Fig. 73-2).7 In 2012, however, the leadership of the Fistula First suggested that the focus of the initiative should likely be “catheter last,” given the high prevalence of dialysis catheter use, particularly among incident patients.8
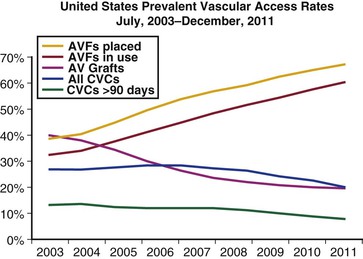
Figure 73-2 The prevalent rates for the various vascular access configurations in the United States are shown for the time period from 7/2003 to 12/2011. Note the dramatic increase in the autogenous [arteriovenous (AV) accesses] (AVFs [AV fistulae] in use) and the corresponding decrease in the rates of prosthetic AV accesses (AV Grafts). The rates of the patients dialyzing with a dialysis catheter have remained relatively stable. CVCs, Central venous catheters. (From the Fistula First: National Vascular Access Improvement Initiative website, http://www.fistulafirst.org/AboutFistulaFirst/FFBIData.aspx.)
The Centers for Medicare and Medicaid Services has implemented a Quality Improvement Program or pay-for-performance initiative that places up to 2% of dialysis center payments at risk.9 This program was implemented in 2012 for the payment year 2014 and is designed to cut reimbursement for dialysis centers not reaching the defined benchmarks. It is based on six quality improvement measures (three clinical and three reporting), including one for the type of vascular access with the access measure based on the percentages of dialysis patients using an autogenous AV access and those using a tunneled catheter.
Society for Vascular Surgery
The Society for Vascular Surgery also assembled a multidisciplinary group and commissioned a systematic review of the literature to examine the timing of referral for vascular access, the type of access to be placed, and the role of access surveillance. The multidisciplinary group then formulated seven practice guidelines.10 Similar to KDOQI and Fistula First guidelines, the Society for Vascular Surgery guidelines emphasized early referral to an access surgeon, the use of autogenous access, and the role of access surveillance and monitoring. However, the systematic review that constituted the basis for the recommendations emphasized the limitations in the supporting evidence. The researchers concluded that “low quality evidence from inconsistent studies with limited protection against bias shows that autogenous access for hemodialysis is superior to prosthetic access.”11 Similar statements qualifying the recommendations for early referral and access surveillance were published in the guidelines. Indeed, there has been some resistance or “pushback” with regard to several of the KDOQI guidelines, as reflected by the evolution from “fistula first” to “catheter last” and the focus on a “functional access” rather than an autogenous access. Hiremath et al12 performed a decision analysis examining the strategy of early referral as recommended by the guidelines and concluded that a “wait strategy” may be associated with greater quality-adjusted life expectancy.
Choice of Arteriovenous Access Type
General Considerations
The requirements for an ideal AV access include a flow rate sufficient for effective dialysis, excellent long-term patency, minimal access-related complications, and a cosmetic appearance acceptable to patients. Unfortunately, no access type fulfills all of these requirements. A mature autogenous AV access satisfies most of these criteria and is likely the optimal access choice, as emphasized by the national guidelines and initiatives previously detailed. Indeed, most developed countries outside the United States have already achieved the KDOQI targets.13 However, it is important to emphasize that the ultimate goal is a functional access with minimal complications, not necessarily an autogenous access.
Patency
Improved patency has been cited as a major justification for the preferential use of autogenous access. The patency rates for upper extremity autogenous and prosthetic AV accesses in adults were defined by Huber et al14 in their systematic review of the literature (Fig. 73-3). Although the primary and secondary patency rates for the autogenous access were superior, the quality of the underlying evidence was limited and likely included a selection and publication bias. Notably, the reported patency rates for both the autogenous and prosthetic accessess were significantly less than the outcome measures defined by KDOQI. Disbrow et al15 compared the patency rates between autogenous and prosthetic AV accesses among incident dialysis patients with suitable veins for autogenous access. The investigators concluded that the patency and intervention rates for the two access types were comparable although the patients with autogenous accesses required the use of a tunneled catheter for a longer period. Schild et al16 reported that the long-term patency rates for prosthetic and autogenous AV accesses were comparable, concluding that prosthetic access should be placed in patients who were not candidates for autogenous access. The secondary patency rates for prosthetic access were 75% at 1 year in a multicenter, randomized trial examining the role of dipyridamole, although a comparable percentage (i.e., 75%) of patients required an intervention to maintain patency.17 Notably, these patency rates were comparable to those reported for autogenous access by Huber et al14 in their systematic review.
Figure 73-3 The patency rates for the autogenous (Auto) and prosthetic (PTFE) upper extremity arteriovenous (AV) accesses are plotted against time (months) with the positive standard error bars. Both the primary (Auto 1, PTFE 1) and secondary (Auto 2, PTFE 2) patency rates for the two access types are shown. The patency rates for the autogenous accesses were better than their corresponding prosthetic counterparts with the one exception of the initial (1.5-mo) time point for the primary patency comparison. PTFE, Polytetrafluoroethylene. (From Huber TS, et al: Patency of autogenous and PTFE upper extremity arteriovenous hemodialysis accesses: a systematic review. J Vasc Surg 38:1005-1011, 2003.)
Autogenous Access Maturation
The construction of autogenous AV access requires an obligatory time for the access to “mature” or to become suitable for dialysis, with a median of 98 days in the United States as reported from the Dialysis Outcomes and Practice Patterns Study.18 During this maturation period, the vein that constitutes the access dilates, the wall thickens (or becomes “arterialized”), and the flow through the access circuit increases. The KDOQI guidelines have defined the “rule of 6s” as the criteria for access maturation and/or suitability for cannulation; they include a vein diameter of 6 mm, an access flow rate of 600 mL/min, and an access depth of 6 mm below the skin.6 Unfortunately, a significant proportion of the autogenous accesses fail to mature to suitability for cannulation. Dember et al19 reported that the autogenous access nonmaturation rate was 62% in the Dialysis Access Consortium (DAC) trial, a multicenter, randomized, National Institutes of Health (NIH) trial examining the effect of clopidogrel on early patency. Similarly, Huijbregts et al20 reported that the implementation of guidelines to increase the autogenous access rate in the Netherlands was associated with a nonmaturation rate ranging from 8% to 50% among the contributing centers. The DAC trial has been criticized for the high nonmaturation rate, the relatively low rate of remedial procedures, and the large number of participating surgeons. However, it was a well-designed randomized, controlled trial conducted primarily at academic medical centers.
This inability of some autogenous AV accesses to mature represents the major limitation. This inability to “mature” can be multifactorial and related to early thrombosis, failure of the vein to dilate, or the inability to cannulate. Review of the DAC trial found that among the accesses that failed to mature, roughly half were abandoned, a quarter were followed up but never cannulated, and a quarter failed cannulation attempts. Some surgeons believe that the greater emphasis on autogenous access from KDOQI and Fistula First has resulted in the unintended consequence of increasing the nonmaturation rate, raising the number of remedial procedures to facilitate maturation (and associated costs), and extending the period of catheter dependence.21,22 The National Institutes of Health recognized the nonmaturation issue identified by the DAC trial and has funded a follow-up study, the Hemodialysis Fistula Maturation Study, to examine the predictors of maturation, including anatomy, biology, patient-specific factors, and processes of care. Rosas and Feldman23 performed an analysis comparing autogenous and prosthetic AV access for incident dialysis patients and found that there were minimal differences in the quality-adjusted life years saved (QALYS), with an autogenous access choice being the dominant strategy if the maturation rate was greater than 69%. Xue et al,24 performing a similar decision analysis among incident dialysis patients, found that autogenous accesses were favored but that the observed differences were less that those suggested by the literature; they concluded that patients with a high risk for autogenous access failure may be better served with other access options.
Morbidity, Mortality, and Cost
The overall morbidity, mortality, and costs associated with maintaining hemodialysis access also strongly favor both autogenous and prosthetic AV accesses over tunneled dialysis catheters, as highlighted previously.25–28 There is likely an incremental benefit in favor of autogenous access over prosthetic accesses in terms of infectious complications, but the differences in terms of the other measures are somewhat equivocal.23,25,26
Role of Prosthetic Arteriovenous Accesses and Catheters
Despite the strong emphasis on autogenous AV access, several advantages of prosthetic access and tunneled catheters merit discussion. Prosthetic accesses afford a large surface area (depending on configuration) and are easier to cannulate than autogenous accesses. Indeed, a survey of provider preferences reported that the dialysis nurses and technicians preferred prosthetic accesses because of the ease of cannulation, whereas physicians preferred autogenous accesses because of the better patency and lower complication rates.29 The obligatory maturation period for prosthetic accesses is shorter (commonly 3-6 weeks), and there are a few commercially available prosthetic accesses that can be cannulated immediately after implantation. Prosthetic accesses are more amenable to remedial therapies in the presence of a “failing” or thrombosed access than autogenous accesses. Additionally, the prosthetic accesses come in a variety of lengths and configurations and there is essentially an unlimited supply. The major advantage of tunneled dialysis catheters is the relative ease associated with insertion (and removal). Indeed, they can provide an immediate life-saving access for the patient without a functional permanent access.
Patient-Specific Considerations
The goal for the individual patient is to establish the most functional access with good long-term patency and minimal complications. As outlined previously, this is an autogenous AV access for most patients. However, a variety of concerns should be addressed in this complex decision process about the most appropriate access for a specific patient, including age, gender, life expectancy, anatomy, comorbidities, likelihood of success (i.e., autogenous maturation rate, long-term prosthetic access patency), urgency of dialysis, and patient preference. In most cases, the question about the most appropriate access can be distilled down to whether the patient is suitable for an autogenous AV access and whether the access is likely to be successful. The important distinction between the emphasis on a functional access and an autogenous access is illustrated by the prospective, longitudinal study by Solesky et al,22 who tracked outcome after access creation. They were able to achieve the national target rate for autogenous access of 66%, but a significant portion of the dialysis support over the study period was provided by catheters, with a 27% catheter prevalence rate. This experience led them to conclude that the current algorithms are problematic and rely heavily on catheters.
Predicting Autogenous Access Outcome
A variety of factors have been identified to predict whether an autogenous AV access will mature and/or maintain patency. Unfortunately, the reports in the literature have been somewhat contradictory and/or inconsistent, and it is the collective hope that the Hemodialysis Fistula Maturation Study, which ended enrollment in August 2013, will provide further insight. In 2012, Smith et al30 published an exhaustive systematic review of the available literature in which they identified several nonmodifiable and modifiable factors. The nonmodifiable factors include increased age, diabetes, predialysis hypotension, artery diameter, arteriosclerosis, vein diameter, and vein distensibility. The modifiable factors include smoking, timing of referral for access, preoperative ultrasound imaging, anastomotic configuration, anastomotic technique, flow assessment, antiplatelet agents, far-infrared therapy, and the timing of cannulation. Notably, gender, body mass index, access surveillance, and the various needle cannulation techniques were not found to be predictive of autogenous access success.
Age.
Advanced age has consistently been associated with autogenous AV access failure, particularly with regard to the radial-cephalic configuration.31–34 Indeed, it has been suggested that the national initiatives may need to be reevaluated for elderly patients.35 Advanced age and life expectancy are interrelated in terms of access choice because the improved long-term patency attributed to autogenous accesses may not be as relevant for elderly patients, particularly if one considers that the obligatory period from access creation to cannulation can be prolonged. Vachharajani et al36 examined the role of the Fistula First Initiative in octogenarians and emphasized that life expectancy and functional status should be considered, given the poor long-term outcome, high mortality, and high discharge rate to a chronic care facility of the patients in the study. Tunneled dialysis catheters may be a reasonable option in some elderly patients.
Diabetes.
Diabetes has also been associated with failure of autogenous access. As for advanced age, the negative predictor for the presence of diabetes has been associated primarily with the radial-cephalic configuration.31,37,38 Indeed, the overall success rate for this configuration has been somewhat poor, particularly in the presence of advanced age, diabetes, and female gender. Why the success rate for diabetic patients is compromised is not completely clear, but the reason is likely related to the characteristic distribution of arterial occlusive disease in the forearm in such patients. Interestingly, diabetic patients have been shown to have comparable success rates for autogenous access procedures in the upper arm and comparable access options as shown by noninvasive imaging.39,40
Obesity.
Obesity has not been shown to be a consistent predictor of autogenous access failure except perhaps for patients in the highest quartile.41 However, the presence of obesity clearly affects the decision about the most appropriate access choice and increases the likelihood of postoperative complications, particularly wound complications. Obese patients have been found to have the same number of autogenous access options on noninvasive imaging.42 However, the cephalic vein, which usually courses superficially, may be somewhat deep relative to the skin and mandate elevation and/or transposition in an obese patient.43
Vessel Characteristics.
The quality of the inflow artery and the outflow vein has consistently been associated with AV access success. Indeed, the quality of the inflow artery has likely been underappreciated as a significant component. The absolute diameter thresholds have been somewhat variable and series dependent. However, arterial diameters smaller than 2 mm have consistently been associated with poor autogenous maturation rates.44–46 This finding is relevant to the radial artery because the brachial artery is consistently above this threshold, even in small women. These threshold radial artery diameters and their associated poor maturation rates likely reflect the presence of forearm arterial occlusive disease and the inability of the forearm vessels to dilate in response to the AV fistula rather than the absolute diameter measurement, because smaller-diameter arteries in children (without atherosclerosis) can frequently yield successful, mature autogenous AV accesses. The early or high bifurcation of the brachial artery (i.e., early takeoff of the radial artery), seen in up to 20% of individuals, has also been associated with lower success rates for both autogenous and prosthetic accesses.47,48 The success rate for brachial artery–based autogenous access have been consistently shown to be greater than those originating off the radial artery, likely reflecting the vessels’ absolute diameters and the distribution of arterial occlusive disease as noted previously.20,49,50 The absolute vein diameter that has been predictive of a successful autogenous access have varied, with minimum criteria ranging from more than 2.0 mm to more than 3.0 mm.51–53 Notably, Mendes et al51 reported that veins with a diameter less than 2 mm were associated with a 16% maturation rate for radial-cephalic accesses. My colleagues and I, at the University of Florida, have used 3.0 mm as our minimal vein diameter criteria for adults, largely on the basis of our experience with lower extremity revascularizations.54
Patient Preference.
Patient preference should also be factored into the decision algorithm about the most appropriate access type or configuration. As already noted, physicians prefer the autogenous access because of its better long-term outcome, whereas the dialysis staff prefers the prosthetic access because the ease of cannulation.29 However, patients prefer a superficial access in the forearm and are more concerned about the ease of cannulation, cosmetic appearance, and impact on daily life than about the distinction between autogenous and prosthetic accesses.29,55,56
Decision Making
The critical question remains as to how individual predictors can or should be incorporated into the selection of the most appropriate access choice for an individual patient. Such a choice incorporates some potentially modifiable (e.g., smoking, specific choice of artery/vein, obesity) and nonmodifiable (e.g., age, gender, presence of peripheral vascular disease) factors. Lok et al57 have developed a scoring system to predict autogenous access maturation. They identified age greater than 65 years (2 pts), peripheral vascular disease (3 pts), coronary artery disease (2.5 pts), and white race (−3 pts) as significant predictors. In their model, patients were given 3 points as a baseline and then the individual scores for the various comorbidities were summed. Successful maturation was found to correlate with the point total, in that patients in the highest-scoring group, with a score higher than 9, had a failure rate of 69% (Fig. 73-4). Notably, a 70-year-old African-American woman (age 2 pts, race 0, baseline 3 pts) with an above-knee amputation (peripheral veno-occlusive disease 3 pts) and coronary artery disease (CAD 2.5 pts) would have a score of 10.5, corresponding to a 31% successful maturation rate. Interestingly, Lilly et al58 used data from the Centers for Medicare and Medicaid Services to examine the Lok model and concluded that the model was not all that predictive, emphasizing that many “high-risk” patients still had successful autogenous accesses. However, their validation study was limited by the administrative database, the lack of the access history, and the inability to validate the data.
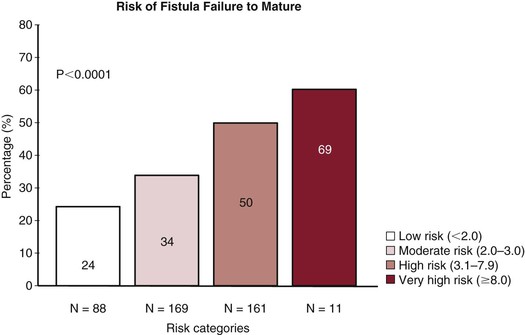
Figure 73-4 The risk that the autogenous access will fail to mature is shown on the basis of the risk categories and scoring system proposed by Lok et al.57 Patients were given 3 points at baseline and then additional points on the basis of age >65 years (2 pts), peripheral vascular disease (3 pts), coronary artery disease (2.5 pts), and white race (−3 pts). The individual scores for the various comorbidities were summed and then broken down into the following risk categories: low risk (<2.0 pts), moderate risk (2.0-3.0 pts), high risk (3.1-7.9 pts), and very high risk (>8.0 pts). (From Lok CE, et al: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17:3204-3212, 2006.)
Lok et al57 have incorporated their scoring system into an initiative entitled “achieve appropriate access” (personal communication, 2012). Their comprehensive approach includes selecting the most appropriate patient on the basis of their scoring system, comorbidities, prior access history, and stage of chronic kidney disease. It involves selecting the most appropriate access for a patient with the least amount of complications by selecting the most appropriate vessels. Furthermore, it involves diligent follow-up with appropriate interventions, all within the context of a multidisciplinary team offering the full range of access options and encompassing experienced surgeons.
The role of prosthetic accesses in this patient-centric decision algorithm about the optimal access choice was further defined by Cull (Table 73-1)59 and reflects many of the issues already identified. In his thoughtful discussion, this writer emphasizes the balance between urgency of dialysis and the likelihood of successful autogenous access maturation as part of the clinical algorithm for selecting an autogenous or prosthetic access. Lacson et al60 have developed an alternative approach, focusing on a “catheter last” algorithm that incorporates expanded use of peritoneal dialysis, use of early cannulation prosthetic accesses, judicious use of autogenous accesses, careful use of existing accesses, and aggressive follow-up with monitoring and surveillance.
Arteriovenous Access Construction
The approach to dialysis patients is similar to the approach to the larger cohort of patients with peripheral arterial occlusive disease, being based on adequate inflow (arterial), adequate outflow (vein), and an appropriate conduit (vein or prosthetic) with a vein conduit being optimal in most situations. Despite some resistance to the national guidelines already outlined, an autogenous AV access is usually the best choice for a functional access, and it has been our experience that it is possible to achieve this end point for most patients, as reported in our prospectively validated algorithm (Fig. 73-5).53 We have relied on a tunneled catheter as a “bridge” until the permanent access is suitable for cannulation in patients with ESRD (as opposed to CKD) and have assumed an aggressive approach to “failing” or “nonmatured” accesses. However, we have made every attempt to limit the use of catheters, in accordance with the national guidelines. Lastly, we have not felt limited by the usual convention of using the nondominant extremity before the dominant extremity or the forearm before the upper arm, rather electing to use the artery and vein combination most likely to result in a functional autogenous access.
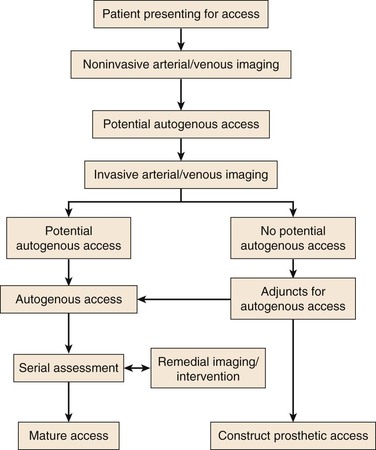
Figure 73-5 An algorithm for patients presenting for permanent hemodialysis access. Note that invasive arterial/venous imaging now includes both catheter- and CT-based arteriography and venography. Patients with no potential autogenous access due to peripheral veins that are insufficient diameter (<3 mm) either undergo re-imaging in the operating room with ultrasound after induction of anesthesia or dissection and direct exploration of the veins. Adjuncts for autogenous access include endovascular treatment of arterial inflow/venous outflow lesions and composite access configurations. (From Huber TS, et al: Prospective validation of an algorithm to maximize arteriovenous fistulae for chronic hemodialysis access. J Vasc Surg 36:452-459, 2002.)
Preoperative Evaluation
Early Referral
The KDOQI recommendations outline the preoperative evaluation for patients requiring permanent hemodialysis access.6 Ideally, patients should initiate dialysis with a functional autogenous access, albeit rarely achieved. This goal requires early referral to an access surgeon and access placement well in advance of the target date so that the access (autogenous or prosthetic) is suitable for cannulation when required, allowing for the requisite time for maturation and remedial interventions. Accordingly, the KDOQI panel recommends that patients with a glomerular filtration rate lower than 30 mL/min/1.73 m2 (chronic kidney disease stage 4) should be educated about the different renal replacement therapies (i.e., transplantation, hemodialysis, peritoneal dialysis). The panel also recommends that an autogenous access be placed at least 6 months prior to the anticipated dialysis start date, and a prosthetic access should be placed 3 to 6 weeks in advance. Unfortunately, it is difficult to predict a specific start date for hemodialysis, and thus, the timing of the access procedures can be imprecise. From a practical standpoint, it is reasonable to construct an autogenous access well in advance of the anticipated start date, given the requirement to achieve maturation and the excellent long-term patency of a mature autogenous access. We usually delay placing a prosthetic access until patients are just about to initiate dialysis because the access can be used shortly after construction and the longer-term patency rate is somewhat limited.
Preservation of Autogenous Options
Patients should be engaged about their imminent need for dialysis, and appropriate precautions should be taken to optimize the number of potential access options. All upper extremity veins that are potentially suitable for an access should be preserved, and both percutaneously inserted central catheters (PICCs) and subclavian vein catheters should be avoided. PICC lines have been reported to cause a central vein stenosis or occlusion in up to 7% of cases and the adverse event rate is as much as 40% for subclavian catheters.61,62 Notably, these central vein lesions potentially preclude an ipsilateral permanent access. In the inpatient setting, we frequently post a sign above the patient’s bed recommending against blood draws or intravenous catheters in the extremity selected for the future access, and we have educated our PICC team and intensivists about the complication of central vein cannulation. Similarly, we have also avoided using arm veins for lower extremity revascularizations in patients with CKD and ESRD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree