Chapter 76
Hemodialysis Access
Failing and Thrombosed
George H. Meier
Although placement of arteriovenous (AV) hemodialysis access is an important step in the management of patients with end-stage renal disease (ESRD), the maintenance and remediation of a failing or thrombosed AV access is almost as important. Dialysis access is a lifeline for ESRD patients, so access maturation and continued function are critical for these patients’ overall well-being. The specific treatments for a nonmaturing or failing AV access may be slightly different from those for a thrombosed AV access; however, the long-term management of all dialysis patients centers on preserving the adequacy of their dialysis treatments for as long as possible with minimal intervention.
The flow disturbances and hemodynamic changes associated with an AV access can initiate an intimal hyperplasia (IH) response (Figs. 76-1 and 76-2).1–3 The IH occurs primarily at the outflow anastomosis of a prosthetic AV access and anywhere along the outflow vein in an autogenous AV access. It can also involve the distant, ipsilateral central veins (e.g., subclavian), even in the absence of previous indwelling catheters.4,5 Some patients develop intractable IH with the early onset of a critical stenosis, whereas others have a more limited response without a significant hemodynamic impact. The underlying mechanisms responsible for AV access IH are ill-defined, but the resulting lesions are common, occurring in the majority of patients with dialysis access, whether autogenous or prosthetic.
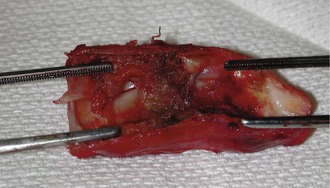
Figure 76-1 Surgical specimen demonstrating typical intimal hyperplasia at the venous anastomosis of prosthetic arteriovenous access.
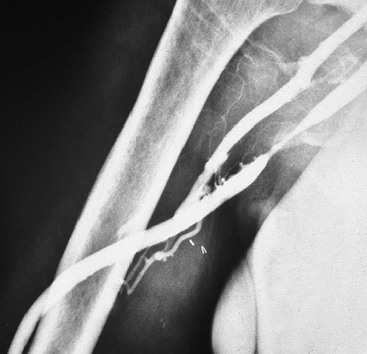
Figure 76-2 Fistulagram demonstrating intimal hyperplasia at the venous anastomosis of a brachial-axillar prosthetic arteriovenous access.
Measuring Access Function
The optimal management of a failing or thrombosed AV access is partially contingent upon detecting access dysfunction. The most common measurement of access function is urea clearance with dialysis, or Kt/V, where K is the rate of clearance of urea, calculated from the pre- and postdialysis measurements; t is the duration of dialysis; and V is the patient’s urea distribution volume. In this fashion, the “dose” of dialysis in a given session can be calculated objectively.6–8
The function or quality of the AV access affects the maximum dialysis dose in several ways. First, adequate flows in the access are needed for the dialysis machine to function efficiently. Currently, high-flow dialysis is the norm, with pump speeds of 350 mL/min or more. As long as the dialysis membranes can tolerate this high flow, the dose of dialysis can be given in a shorter time, reducing both costs and resources. Nonetheless, this high-flux technique requires much more efficient access function and is less tolerant of problems related to recirculation or high venous pressures. Recirculation, the retreatment of blood already filtered by the dialysis machine, can be insidious, often showing up as poorer solute clearances with each subsequent dialysis treatment. Pressure limits are typically set on the dialysis machine; as a result, increasing venous pressures result in more prolonged dialysis because of efforts to reposition the efferent needle, and thus, remedy the high pressures.
Mechanisms of Access Failure
Flow Limitation
A functional AV access requires flow rates that exceed the pump speed of the dialysis machine by several-fold. With modern, high-flux dialysis, pump speeds may approach 500 mL/min. Thus, flows of 1000 to 1200 mL/min are needed in the AV access to avoid recirculation.9 Additionally, adequate cardiac output is essential to maintain these flow rates. If cardiac output is marginal, decreased access flow may occur over the course of the dialysis session because of decreased preload associated with successful fluid removal. Limitation of the access flow, by whatever mechanism, results in the recirculation of already dialyzed blood to the dialysis machine, greatly limiting the effectiveness of dialysis.9–15 Recirculation can result when the afferent needle pulls blood that has just been returned to the patient via the efferent needle. This partially dialyzed blood is then dialyzed a second time, with decreased removal of substrate caused by lower concentrations in the partially dialyzed blood. This results in a decreased effective dialysis dose, with a longer duration of dialysis required to achieve the same clearance. There are several mechanisms by which recirculation can occur.
Venous Outflow Stenosis.
The classic cause of recirculation is venous outflow stenosis, resulting in decreased flow through the AV access and increased recirculation of blood to the afferent needle. Regardless of the adequacy of arterial inflow, the outflow stenosis limits flow and increases recirculation. This is a common problem seen with failing prosthetic AV accesses.
Arterial Inflow Stenosis.
Arterial inflow stenosis can similarly limit flow through the AV access, but in this case, both needles are distal to the stenosis. The limited arterial inflow results in recirculation from the distal efferent needle to the proximal afferent needle. This is a common mechanism of failure of autogenous AV access, occasionally leading to complete collapse of the outflow vein during dialysis.
Cannulation Location.
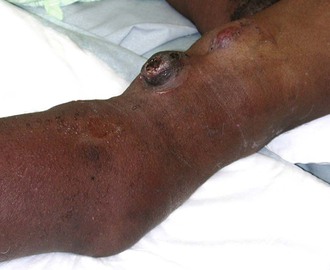
Another important cause of recirculation is inadequate separation of the cannulation needles. Specifically, recirculation will occur if the needles are positioned too close together, even in the presence of a high access flow rate. Repeatedly puncturing the access at the same convenient location, rather than rotating puncture sites, can cause false aneurysms or pseudoaneurysms. If these are sufficiently large, recirculation can occur because of the stagnant flow within the pseudoaneurysm. Additionally, these pseudoaneurysms are problematic because they are often the sites of infiltration and bleeding (Fig. 76-3).
Conduit Access Limitation
Although access flow is of fundamental importance, the conduit itself may be the cause of problems, or there may be secondary issues that limit the ability to puncture the AV access for reliable dialysis. If the vein is too deep or too small for reliable cannulation, dialysis may be problematic, even with adequate flow. In a morbidly obese patient with ESRD, a vein of any size may be inadequate because of the depth of the overlying skin and soft tissue through which the vein must be cannulated.16–18 In these patients, superficial transposition or elevation of the vein conduit may be necessary to allow maturation and reliable cannulation.19–23
Causes of Access Failure
Once dialysis access is established, the mode of failure is usually related to the type of access constructed. In the case of tunneled dialysis catheters, failure usually results from thrombus or the development of a fibrin sheath surrounding the catheter (see Chapter 74). For both autogenous and prosthetic AV accesses, the issue is often the development of venous outflow stenosis or stenosis of the autogenous access itself resulting from IH, leading to limited clearance.24–29 Autogenous AV accesses have limited patency after thrombectomy, so intervention is recommended before thrombosis occurs. Prosthetic accesses thrombose at a higher overall flow rate than autogenous accesses, but they have better patency after thrombectomy. Thrombosed prosthetic accesses can often be remediated multiple times before they need to be abandoned.
IH is one of the unresolved issues affecting both the establishment and maintenance of a functional AV access. Although IH can occur anywhere in the outflow veins, certain anatomic factors can predispose to local or remote stenoses. For example, central venous stenosis is common in both autogenous and prosthetic AV accesses (Fig. 76-4). In the past, this was often attributed to the use of subclavian catheters, because the subclavian veins were the most common sites of central venous access.30 In most modern dialysis centers, use of the subclavian veins for catheter access is avoided, specifically for this reason; yet there is still a high incidence of subclavian vein stenosis with upper extremity accesses. It is likely that the high flow across the subclavian vein at the thoracic inlet generates a perturbation in flow that leads to IH and stenosis. In this fashion, almost any vein used in the outflow of an AV access can develop IH and stenosis. Another unavoidable factor is puncture of the conduit that leads to IH in autogenous conduits and to local tissue ingrowth in prosthetic accesses, simulating IH. Finally, IH occurs at the venous anastomosis of prosthetic AV access, theoretically aggravated by the excess turbulence associated with the size and compliance mismatch between the prosthetic graft and the outflow vein (see Chapter 6).
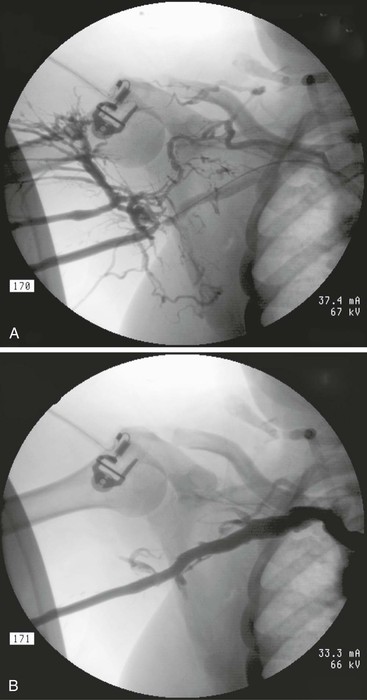
Figure 76-4 A stenosis in the axillary vein secondary to intimal hyperplasia is shown (A) before and (B) after successful treatment with an 8-mm high-pressure angioplasty balloon. Note the resolution of the venous collaterals.
Detection of Access Failure
Clinical Evaluation
In many cases, clinical examination may be sufficient to assess access function with a reasonable degree of certainty. If an access is functioning well, a continuous thrill should be present near the arterial anastomosis, and it should be detectable for several centimeters over the outflow vein. If a pulse is present near the venous outflow, venous outflow stenosis is likely. In most cases, a thrill distal to an area of pulsatility suggests the location of the stenosis. Pseudoaneurysms of the access may result in local pulsatility in the areas of enlargement, independent of venous outflow issues. Only areas of normal diameter can be assessed by physical examination for pulsatility caused by venous outflow stenosis; aneurysmal or pseudoaneurysmal areas will often have increased pulsatility.
Collateral Veins or Edema.
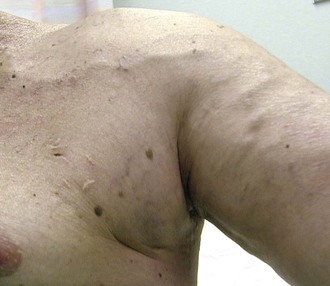
Collateral veins around areas of stenosis or occlusion are important indicators of elevated venous pressures (Fig. 76-5). Typically, this occurs around the shoulder or the anterior chest wall for upper extremity accesses as a result of a subclavian vein stenosis or occlusion. Thus, the physical examination in these patients should include special attention to the head, neck, chest, and shoulder. Similarly, patients with a significant venous outflow stenosis without adequate collaterals may present with upper extremity edema alone.4,31,32
Access Bleeding.
High venous pressures can lead to excessive access bleeding after removal of the cannulation needles, and this is often discovered by the dialysis center staff because of the prolonged time required to obtain hemostasis.33–36 This may be the first indication of increased venous pressures and is a common indication for interrogating the access with either duplex ultrasound or venography. Venography may be better than ultrasound in this situation because it affords the opportunity to both diagnose and treat any venous outflow lesion (see section on Interventions for Failing Access). The central vein stenoses responsible for the elevated pressures can lead to an insidious decrease in the access flow rates, often not recognized until later by the dialysis staff. Thus, excessive access bleeding should initiate an evaluation that can lead to intervention before access failure; this is especially important for autogenous AV access.
Failure to Mature
The increased blood flow through the outflow vein of an autogenous AV access causes the vein to dilate and its wall to thicken or become “arterialized.” Although this process varies by patient and specific vein, the outflow vein usually matures sufficiently for cannulation within 3 to 4 months of creation. When an autogenous AV access fails to mature, the underlying causes may be difficult to distinguish on clinical examination. In the presence of an arterial inflow stenosis, a good thrill may be present, but inadequate flow through the narrowed segment may prevent reliable cannulation with the venous return needle (i.e., efferent needle). Cannulation can be assisted by occluding the venous outflow using a tourniquet to allow maximum venous distention for the puncture; however, any branches close to the site of stenosis may prevent distention and lead to an inability to cannulate the access. These collateral or “accessory” veins can also theoretically inhibit the maturation process of the main outflow vein by the diversion of blood away from the main channel, even in the absence of an arterial inflow stenosis. It is somewhat paradoxical that low resistance venous outflow improves dialysis function by preventing recirculation, although it can make access cannulation more difficult.
Inability to reliably cannulate an autogenous AV access for dialysis may also indicate that an access has failed to mature and may require revision, despite the fact that the vein may have dilated sufficiently (i.e., 6 mm). It is important to emphasize that the ability to reliably cannulate an access is also dependent on the experience of the individual performing the cannulation in addition to the quality of the access itself. Fragile, immature veins that have not become arterialized sufficiently to withstand the trauma of repeated cannulations may lead to hematomas around the needle access site. The presence of significant hematomas may lead to further problems cannulating the access (both autogenous and prosthetic) and predispose to the development of a stenosis. In other cases, the access vein may simply be too deep to allow easy cannulation. An open dialogue between the access surgeon and the dialysis center personnel is important to determine the cause of any cannulation difficulties to both optimize maturation and prolong the functional life of the access.
Assessment During Dialysis
There are several techniques for monitoring dialysis access function, all based upon the measurements of access flow, pressure, or flow resistance. From a practical standpoint, two methods predominate: measurement of static venous pressure, and measurement of flow rate using ultrasound flow measurement.
Venous Pressure Measurement.
Static venous pressure is perhaps the easiest of the surveillance techniques and the most widely used in dialysis centers.35,37–39 To measure this value, the dialysis pump is turned off, and the circuit is allowed to equilibrate. The venous (i.e., efferent) needle pressure is then used as the static venous pressure. Individual measurements that are greater than 50% of the mean arterial pressure are considered abnormal; however, the trends over time are more reliable and predictive than a single measurement. Specifically, if the static venous pressures increase over a series of measurements, investigation is indicated even if the 50% mean arterial pressure threshold is not achieved.
Flow Measurement.
The second technique for assessment is ultrasound velocity dilution using a Transonic Hemodialysis Monitor (Transonic Systems Inc., Ithaca, NY) within the dialysis circuit.40–45 Using this technique, a bolus of isotonic saline is injected into the blood stream and dilutes the blood and the corresponding ultrasound velocity. As the saline passes through the blood lines, a sensor registers an indicator curve that can be used to calculate the flow rate. There are several advantages of this technique. First, flow is measured by saline dilution with the needles reversed, producing a stable measure of access flow that is independent of the volume of the saline injected. Second, access recirculation can be assessed objectively at the same time with the same equipment using the needles in their normal orientation.42,46–49 Therefore, access flow and access recirculation can be assessed repeatedly over time to provide two independent measures of access function.
The development of an access stenosis is highly variable in onset. Some patients develop clinically significant stenosis within a few weeks of access creation, whereas others never develop a stenosis. Generally, a flow rate of less than 600 to 800 mL/min predicts thrombosis in a prosthetic access, although the thresholds for autogenous accesses are not well defined.10,50–52 The National Kidney Foundation Kidney Disease Outcome Quality Initiative (KDOQI) Clinical Practice Recommendations for Vascular Access recommends further study and intervention when this threshold is achieved.53 However, there is some suspicion that these rates may be too low, and that interrogation or intervention may be appropriate at a higher level (e.g., flow rates <900 mL/min may justify evaluation). The KDOQI also recommends at least a monthly assessment of access flow using any number of techniques.53
Duplex Ultrasound Access Surveillance
Although duplex ultrasound surveillance has been attempted for AV access, there are currently few data to suggest that it provides any real benefit in maintaining access function long term. Prospective trials of duplex ultrasound surveillance have failed to demonstrate any patency benefit.54 Duplex ultrasound can be useful in confirming any suspected abnormalities in access flow detected by physical examination, although it may not add much beyond a thorough examination by an experienced clinician. For this reason, duplex ultrasound for AV access is generally reserved for those situations in which the clinical examination is confusing or difficult.
Catheter-Based Contrast Imaging
Catheter-based contrast imaging is essential to any intervention to maintain or improve dialysis access function. Contrast imaging is, by definition, invasive because needle or catheter access involves skin puncture. Generally, contrast imaging provides anatomic information rather than physiologic data, although access flow can be inferred from the anatomic images. For this reason, surveillance and monitoring are complementary to contrast imaging, with anatomic imaging providing supporting evidence for the alteration of access function identified by monitoring techniques.57 Additionally, once the underlying anatomic defects are identified, intervention can be performed in the same setting. Performing the diagnostic and therapeutic procedures at the same time can avoid the risk of access failure during the intervening time associated with a staged approach.
Interventions for Failing Access
Open Surgical Techniques
Revision for Stenoses
Open, surgical revision of a stenotic segment within the AV access can improve both flow and function. The challenge is to define the underlying lesion responsible for the diminished flow. As with all access interventions, complete imaging from the arterial inflow to the central veins is necessary, and this is generally done at the time of operative revision. The advantage of simultaneous imaging is that questions concerning the access can be answered in “real time.” Repeat imaging is often necessary to further define the relevant anatomy when the diagnostic imaging is performed at an earlier time.
Surgical revision for stenosis is generally performed using an interposition graft or a patch angioplasty. Both techniques have advantages and disadvantages, can be effective, and are widely used. The use of an interposition graft to bypass a stenosis at the venous outflow of a prosthetic access essentially re-sites the venous anastomosis, thereby limiting the length of available vein that can ultimately be used to construct a permanent access. Patch angioplasty simply enlarges the area of stenosis without removing the local disease process (or consuming any available length of the vein), theoretically leading to a higher incidence of recurrent stenosis. However, results of these two techniques are largely equivalent.58,59
Revision for Other Problems
The presence of multiple outflow vein branches can limit the available vein length for cannulation and can inhibit the dilation of the main outflow vein during the maturation period. The significance of the collaterals may not be obvious at the time of initial access placement, but may become apparent if the access fails to enlarge above a large branch. In this situation, ligation of collateral branches may help maximize outflow through a single axial venous segment.
Translocation or elevation of a deep autogenous AV access may be necessary to allow successful needle cannulation. This is a common problem, particularly in obese patients with type 2 diabetes. In these patients, overlying fat and subcutaneous tissue may limit the ability to cannulate the autogenous access, even in the presence of high flow rates and a good outflow vein. Transposition to a more superficial location may allow access utilization that would otherwise be impossible.
The technique for superficial transposition is similar to that used in the second stage of a two-stage basilic vein transposition. In this situation, the depth of the vein from the skin is generally greater than its diameter. To reliably access the vein, it should be within one vein diameter from the skin surface or within 6 mm as recommended by the KDOQI “rule of 6s,” which is among their criteria for autogenous access cannulation (i.e., vein diameter—6 mm, depth from skin—6 mm, flow rate—600 mL/min).53 There are two basic approaches to making the vein accessible. The first is to remove the fat and subcutaneous tissues overlying the vein and then re-approximate the subcutaneous tissues, which produces a shallower tissue depth to cannulate the access. The challenge in this technique is to somehow avoid developing scar tissue in the subcutaneous space that may limit needle access or generate IH. Bronder et al59 reported favorable results with this technique in a large series of patients.
The second technique involves transection of the vein, with superficial transposition and re-anastomosis. Although it may be possible to simply reattach the vein to its previous anastomosis, the access surgeon should be prepared to re-site the arterial anastomosis more proximally to accommodate any loss of vein length resulting from the new, superficial tunnel. This secondary surgical procedure, although generally not a major operation, may result in significantly greater morbidity than that associated with the original access procedure, given its potential for wound complications in obese patients. After transection of the vein, the longer segment is tunneled in a subcutaneous course. The vein is then carefully re-anastomosed to avoid unnecessary tension. As with the two-stage basilic vein transposition, the vein has already adapted to arterial pressure and flow, making the surgery safer and easier because the vein is somewhat easier to manipulate, although some venous hypertension is associated with the arterial communication. Any other abnormalities in the access can be addressed at the same time. In some cases, during maturation the vein may dilate and elongate, such that simple elevation without transection can be performed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree