Chapter 77
Hemodialysis Access
Nonthrombotic Complications
Linda M. Harris
Based on a chapter in the seventh edition by Hasan H. Dosluoglu and Linda M. Harris
The National Kidney Foundation Kidney Dialysis Outcomes Quality Initiative (KDOQI) has published guidelines (updated in 2006) for optimal clinical practices aimed at improving dialysis outcome and patient survival.1 The reporting standards recognize eight categories of complications related to hemodialysis access: thrombosis and failure to mature (see Chapter 76), bleeding, infection, aneurysm and pseudoaneurysm, seroma, ischemia or steal syndrome, venous hypertension, and neuropathy.2 Cardiopulmonary complications are also addressed in this chapter because they can affect patient outcomes. Catheter complications are dealt with in Chapter 74. The physician establishing hemodialysis access or managing access should be knowledgeable about the potential complications and appropriate interventions.
Bleeding
Patients with end-stage renal disease (ESRD) have an increased risk of bleeding due to defects in hemostatic mechanisms secondary to uremia or acquired or inherited coagulation abnormalities. Bleeding can be a problem during arteriovenous (A-V) hemodialysis access creation or revision as well as during other major operations. Prolonged needle puncture bleeding is another relatively common problem in hemodialysis patients. The reporting standards grade the severity of bleeding 0 to 3, as follows:
Grade 1: Resolves without treatment
Grade 2: Medical therapy needed to correct coagulation abnormality
Etiology
Morgagni in 1764 was the first to recognize the association between kidney dysfunction and a bleeding tendency.3,4 Riesman confirmed the observation in 1907, describing Bright’s disease.5 Patients on hemodialysis also have periodic heparin exposure. Subdural hematoma, hemopericardium, gastrointestinal bleeding, and bleeding into the anterior chamber of the eye, parathyroids, retroperitoneum, and mediastinum have been described in patients on chronic hemodialysis.5 Sood and associates analyzed the U.S. Renal Data System database and found that the incidence of subdural hematomas had more than doubled—from 90 per 100,000 dialysis patients per year in 1991 to 191 per 100,000 dialysis patients in 2002—without a concomitant increase in those undergoing peritoneal dialysis.6 This was attributed to increased warfarin use. Randomized controlled trials have failed to show any benefit of low-intensity warfarin7 or antiplatelet agents8 for preservation of vascular access patency but have shown increased bleeding complications.
There is no direct relationship between the degree of azotemia and bleeding, but as a general rule, the risk of bleeding increases markedly when the blood urea nitrogen concentration exceeds 100 mg/dL (35.7 mmol/L). β-Lactam antibiotics (penicillins and cephalosporins) have a half-life that is prolonged in renal failure and at high levels can cause platelet dysfunction, further increasing risk of bleeding. At extremely high serum levels, penicillins alter antithrombin III activity, causing heparin-like abnormalities.9 Low-molecular-weight heparins are eliminated mainly by the kidneys, and their dosage in hemodialysis patients can be difficult to adjust.10 Skin puncture bleeding time is a reliable predictor of clinical bleeding in the setting of uremia.11,12
The defects in hemostasis in the setting of uremia are multifactorial. Although chronic anemia is one cause, other factors play an important role. At normal hematocrit levels, red blood cells occupy the center of the bloodstream, with platelets and plasma more concentrated at the periphery and able to react readily with the endothelium. In anemia, the rheology of the blood changes and it acts more like a classic newtonian fluid, with platelets and red blood cells evenly mixed.11,13,14 Most research has pointed to platelet dysfunction as the most prominent defect in uremia.3,4,15,16 Platelets of patients with uremia exhibit a decrease in glycoprotein (GP) Ib,17 the receptor for von Willebrand factor (vWF). Furthermore, the GP-IIb/IIIa function of platelets is impaired, most likely the result of a conformational change and a fibrinogen-ligand binding defect of GP-IIb/IIIa.16 Excessive nitric oxide production by uremic vessels, perhaps secondary to uncleared guanidinosuccinic acid, has been implicated as the factor inducing changes in platelet function.4 The final defect seen in uremic patients is the increased endothelial production of prostaglandin I2, a vasodilator with antiplatelet effects.5,13
Although no long-term randomized controlled trials have evaluated the efficacy of aspirin or antiplatelet agents for cardiovascular or stroke prophylaxis in the hemodialysis population, the National Kidney Foundation has recommended aspirin for hemodialysis patients who have or are at high risk for development of cardiovascular disease.18 Holden and associates found the rate of major bleeding episodes, most of which were gastrointestinal in origin, to be 3.1% per person-year in patients taking warfarin, 4.4% in those taking aspirin, and 6.3% in those taking both aspirin and warfarin and pointed out the need for randomized trials to evaluate the efficacy and safety of these agents for secondary prevention of cardiovascular events in hemodialysis patients.19
Access site bleeding after dialysis may be due to platelet dysfunction and anticoagulation received during dialysis; however, it may also be associated with increased venous pressure secondary to venous outflow stenosis or pseudoaneurysms with thin skin and repeated puncture. Physicians should consider these as potential causes, especially if this is a recurrent phenomenon.
Treatment
Treatment of bleeding in hemodialysis patients can be addressed by several means. Adequate dialysis itself can improve platelet function. Maintenance of an adequate hematocrit by the administration of erythropoietin both provides a margin of safety should there be any bleeding and favorably affects the rheology of the blood, thereby facilitating platelet function. Recombinant human erythropoietin also induces an increase in GP-IIb/IIIa expression.16
Intraoperative Bleeding
Intraoperative or postoperative bleeding can be dealt with by administration of 0.3 to 0.4 µg/kg of desmopressin (DDAVP). The DDAVP should be diluted in saline and administered as a short (30-minute) infusion.5 DDAVP releases factor VIII–vWF from storage sites into the plasma and increases the proportion circulating as large multimers.20 The effect of DDAVP should be apparent in 30 minutes and lasts up to 8 hours. Tachyphylaxis to DDAVP typically develops after the second dose, once stores of factor VIII–vWF are exhausted.21 DDAVP may act by transiently decreasing protein C activity as well.3
Cryoprecipitate can also be administered intraoperatively or postoperatively to control acute coagulopathic bleeding. Ten units will have an effect lasting approximately 24 hours.20 It contains large amounts of factor VIII–vWF multimers and fibrinogen. Persistent oozing during surgery or after dialysis may be secondary to continued effects from heparin. If the activated clotting time is elevated, protamine can be administered up to 1 mg/Unit of heparin to reverse the anticoagulant effect. Activated factor VII can be used if all the previously discussed measures fail to arrest the bleeding, but it carries the risk of systemic thrombosis.10
Chronic Treatment
The use of transdermal estradiol is safe and effective in providing longer procoagulant effects.22 Conjugated estrogens can be used with the same effect at a dose of 2.5 to 25 mg orally or 0.6 mg/kg intravenously.13 It is thought that estrogens work by antagonizing the synthesis of nitric oxide.23 The effect of estrogens can be seen within 6 hours24 but is not manifested fully for 5 to 7 days, with effects lasting up to 14 days.13
Strategies to avoid bleeding preemptively include discontinuing aspirin or nonsteroidal anti-inflammatory drugs for 1 week before surgery and performing surgery 24 hours after dialysis to allow the recovery of platelet function. Patients facing major surgery should be prescribed transdermal estrogen (100 µg/24 h) for 2 weeks before the procedure. Intravenous estrogen can be used if a more urgent operation is required.14 Hemodialysis patients are often malnourished to some degree,21 and supplemental vitamin K can be administered as needed. Use of recombinant human erythropoietin should be universal.
Postoperative Bleeding
In most cases, postoperative bleeding should be addressed by return to the operating room and exploration of the site for surgically correctable causes, such as suture line bleeding. The pharmacologic adjuncts described earlier, particularly the use of protamine sulfate to reverse the effects of heparin, should be considered if the initial procedure involved heparinization. DDAVP should also be used liberally to improve platelet function, but not as a substitute for surgical exploration.
Problem bleeding from access puncture sites can be significant enough for patients to seek attention in the emergency department. However, it rarely requires operative intervention. Bleeding should be controlled by applying direct pressure over the point of blood loss. The pressure should be enough to stop the bleeding without obstructing blood flow through the access itself; this usually controls bleeding within 30 minutes or less. Rarely, superficial sutures may be necessary. If the patient has just finished a dialysis session, protamine sulfate can be used to counter any remaining heparin effects. DDAVP can also be helpful. If bleeding after dialysis is a consistent problem, a venous outflow obstruction should be considered and investigated with fistulography or venography.
Infection
Infection is the second leading cause of access loss, after thrombosis, and infectious complications of all types are the second leading cause of death in dialysis patients, second only to cardiac mortality, accounting for 15% to 36%.25–27 In addition, the risk of death due to sepsis is estimated to be 100- to 300-fold higher in patients with ESRD.27 Impaired humoral and cellular immunity, nutritional deficiencies, and type of vascular access are among the major determinants.28 The Society for Vascular Surgery reporting standards recommend classifying the infections as early (<30 days) versus late (>30 days) and culture positive versus negative, with identification of the site of the infection (i.e., para-anastomotic, mid-A-V access, outflow veins). Specific treatment of infections depends on the type of access, the location and extent of infection, and whether the infection is early or late after access creation. Infection is common in prosthetic A-V accesses but can also be seen in autogenous A-V accesses. The grading system facilitates communication of the severity of infection:
Grade 1: Resolved with antibiotic treatment
Grade 2: Loss of A-V access because of ligation, removal, and bypass
Bacteriology
Dialysis-related infection is caused by a predominance of gram-positive organisms; Staphylococcus aureus is the most common isolate. Gram-negative organisms account for 25%, and a smaller percentage are polymicrobial.29,30 The implications of infection with S. aureus are profound, with one study finding complications in 44% and a mortality rate of 14%. Endocarditis, osteomyelitis, and septic arthritis were the most common complications. Because of the risks associated with A-V access infections, broad-spectrum antibiotics should be initiated as soon as access-related infection is considered. Most commonly, vancomycin and gentamicin are chosen because of their broad spectrum and ease of dosing during hemodialysis therapy. Accordingly, hemodialysis patients are at risk for the development of vancomycin resistance.31 In centers where the prevalence of methicillin-resistant S. aureus is low, nafcillin, oxacillin, or cefazolin should be used in place of vancomycin.32,33
Catheter Related
Catheters are the most common site for infection in dialysis patients and are discussed in Chapter 74. The Centers for Disease Control and Prevention performed surveillance of 800 dialysis patients and found that independent risk factors for access infections included the use of a catheter, specific dialysis units, and malnutrition (albumin <3.5 g/dL).34 A study of S. aureus infections in hemodialysis patients demonstrated that 67% of all the access-related infections occurred in patients with a catheter.35 Stevenson and associates27 looked at their experience from 111,383 dialysis sessions during a 2-year period and found a 0.4% rate of access infections. Tunneled catheters accounted for 57% of infections and 73% of the bloodstream infections (relative risk, 13.6). Nontunneled catheters accounted for 10% of the total infection rate, despite being used in only 2% of patients (relative risk, 32.6), whereas prosthetic A-V access had a 2.2 relative risk.27
Epidemiology
The average life span of a prosthetic A-V access is 2 years, with 20% lost as a result of infection.36 A prospective Canadian study showed that the probability of an autogenous A-V access infection is 4.5% in 1 year versus 19.7% for a prosthetic A-V access.37 Jaar and colleagues conducted a longitudinal cohort study of 4005 hemodialysis patients using U.S. Renal Data System data and found a relative risk for infection of 1.35 for prosthetic A-V access versus autogenous A-V access.28 Marr and associates35 reported a 5% annual infection rate from punctures and 3% perioperative wound infection rate in prosthetic A-V access.
Several factors can affect infection risk for access procedures. Repeated cannulation, poor personal hygiene, increased number of hospitalizations, increased duration of prosthetic A-V access use, increased age, diabetes mellitus, and ambulatory limitations have been reported to contribute to prosthetic A-V access site infections.35 Location in the lower extremity was also found to increase the infection risk for prosthetic A-V access compared with upper extremity or autogenous lower extremity A-V accesses38 (18% vs 1.6%; P < .05). Gram-negative bacteria and associated remote infections or complications were also more common with lower extremity prosthetic A-V accesses (31% vs 4%; P = .003).39,40 Access cannulation technique has also been implicated. Whereas use of the buttonhole technique (i.e., cannulation at the exact same site, angle, direction, and depth, with blunt needles used after the track has been developed) has been shown to decrease the rate of aneurysm formation and hematoma, it is associated with higher infection rates than the rope-ladder technique (i.e., use of the entire length of the access, moving 2 cm from last insertion) or area technique (i.e., cannulation of only a few areas) of access. The increased rate of infection has been shown to be related to inappropriate use of sharp needles (which creates new tracks), inadequate use of disinfecting agents, and incomplete scab removal in a study by van Loon et al.41,42
Treatment
Autogenous Access
Infections involving autogenous A-V access can be manifested as diffuse cellulitis or focal abscess and frequently are associated with the cannulation technique or hematomas. These infections rarely require revision or excision of the access. Most respond to a 2- to 4-week course of antibiotics; those with abscess require drainage in addition to antibiotics. However, autogenous A-V access with intraluminal endovascular devices, whether covered or bare metal stents, require a prolonged course (4 to 6 weeks) of parenteral antibiotics or surgical revision.27 Recurrent infections may require ligation or excision of the access. The bacteriology of autogenous A-V access infection does not appear to differ from that of prosthetic A-V access infections.
Prosthetic Access
Treatment of prosthetic A-V access infections may require complex, difficult clinical decisions. Despite the risk of recurrent infection, access salvage is reasonable for limited infections as it allows continued dialysis access without loss of a site, avoids additional catheter days, and decreases complexity of surgical procedures. If only local signs of infection are seen without skin breakdown or bacteremia, salvage may be attempted. Failure to improve requires excision of all unincorporated prosthetic material. Palder and colleagues in 1985 showed that in more than half of the cases of prosthetic A-V access infection, only a discrete portion of the prosthetic material was involved.43
Midgraft Infection.
Salvage of the prosthetic A-V access may be attempted in the presence of localized erythema or a focal sinus track. In these settings, the uninvolved segments of the prosthetic material are exposed proximally and distally (i.e., remote from the region involved with the infection) while the infected region is covered with an impermeable dressing. A new segment of prosthetic material is tunneled through clean tissue planes, the anastomoses are completed, the incisions are closed, and dressings are applied. A separate incision is then made over the infected segment of the prosthetic graft and all involved material removed, thereby isolating the noninfected from the infected segments of the procedure. The infected wound is typically left open and packed. Schwab and coworkers reported 17 cases of immediate graft salvage for infected prosthetic A-V accesses,44 with 94% early success, but noted episodes of recurrent infection in portions of the remaining grafts during follow-up. Raju reported a 90% access salvage rate in a smaller series using the same technique of replacing and rerouting the infected graft segement.45 However, Ryan and associates46 reported worse outcomes with 74% access salvage and wound healing; 26% required conversion to total graft excision because of reinfection or nonhealing wounds. In the largest series, Schutte et al described 91 patients with a 91% success rate, with secondary procedures needed in 19% and a local infection rate of 20%.47
Anastomotic Infection.
Infections involving the anastomosis require complete excision of the prosthetic material because of the risk of anastomotic disruption and hemorrhage.48 Patch repair of the artery can usually be accomplished with a small segment of the outflow vein. This should be done cautiously, as use of an infected segment of vein may potentially predispose to further complications. Alternatively, subtotal graft excision, leaving a small prosthetic cuff on the underlying artery, has been suggested as a means to decrease complexity of the procedure when the involved organisms are relatively nonvirulent (e.g., Staphylococcus epidermidis).48 However, this is associated with a 17% infection rate of the retained cuff.48 Total or subtotal prosthetic graft removal usually leads to abandonment of the site. Brachial artery ligation has also been reported in critically ill patients with grossly infected prosthetic grafts although predictably associated with hand ischemia.49 Replacement of the infected prosthetic A-V access with cryopreserved femoral vein or femoral artery is an alternative to preserve the access site. Matsuura and associates50 reported their experience with 43 patients in whom the infected prosthetic graft was replaced by a cryopreserved femoral vein graft; 32 were tunneled parallel to the previous tunnel, and 11 were placed in the infected field. One-year primary and secondary patency rates were 42% and 68%, with only one recurrent infection (2.3%). However, caution should be exercised with implantation of any nonautogenous tissue into the infected field because of the risk of recurrent infection.
Any significant infection occurring within the first several days after initial graft placement should be treated by complete graft excision and placement of a new access elsewhere.27,44 Vallet and associates51 reported their experience in four patients with early and late infections, as defined by the reporting standards, with exposed prosthetic grafts, not involving the anastomoses, using a vacuum-assisted closure device in addition to débridement and intravenous antibiotics. They achieved a prosthetic access salvage in all patients without need for temporary access, although the role of this modality needs to be further defined.
Thrombosed Grafts.
Unresolved is the issue of what to do with thrombosed, abandoned prosthetic A-V accesses in the face of persistent bacteremia. Removal of a thrombosed, noninfected incorporated prosthetic A-V access has not been considered necessary52 and can be somewhat technically difficult. However, a few studies suggest the potential for the prosthetic material to be a source of infection. A group from Miami reported that 23% of 87 infected prosthetic grafts removed were thrombosed.48 Another study found that 15 of 21 asymptomatic patients had positive indium scans at the site of abandoned access, and subsequent removal of these prosthetic grafts revealed that 13 had perigraft purulence.52 The authors concluded that in the face of a fever of unknown origin in patients with a thrombosed prosthetic A-V access, an indium scan should be obtained, and the access should be removed if the study shows positive uptake.
Pseudoaneurysm
Pseudoaneurysms can occur at autogenous or prosthetic A-V access anastomoses or puncture sites. They are associated with an increased risk of thrombosis, pain, cosmetic problems, infection, bleeding, and difficulty with access.53 Although pseudoaneurysms may be infected, many that develop later are not. Pseudoaneurysm formation in prosthetic access is relatively uncommon but well documented, occurring in 2% to 10% of all cases.54 Poorly incorporated prosthetic A-V accesses or those that are subjected to lacerations from large needle punctures may develop perigraft hematoma or pseudoaneurysm.54 Autogenous A-V accesses may also develop pseudoaneurysms, but at a lower rate than their prosthetic counterparts.
The method of access cannulation may have an impact on development of pseudoaneurysms. Pseudoaneurysms associated with cannulation areas are caused mainly by repetitive single-site needle sticks55 and should lead to abandonment of the area and strict enforcement of rope-ladder cannulation if a buttonhole is not practical. These pseudoaneurysms are commonly combined with stenosis of the access proximal or distal to the aneurysmal segment. Progressive enlargement of the pseudoaneurysm can eventually compromise the overlying skin, leading to rupture and sometimes resulting in death. Large aneurysms can also interfere with cannulation of the adjacent access, limiting the potential cannulation sites. In a study by van Loon et al,41 the authors found that pseudoaneurysm formation is more common with the rope-ladder technique of access cannulation compared with the buttonhole technique. This is thought to be related to multiple different angles of insertion of the needles, potentially causing damage to varying areas of the A-V access. The rope-ladder technique of cannulation involves systematic rotation of the puncture sites of cannulation 2 cm above the previous insertion site, using the entire length of the access. The buttonhole technique, used only for A-V fistulae, requires initial use of sharp needles during the first few weeks, with intentional use of the same angle, direction, and depth to form a track. After a track has formed, subsequent cannulation is performed with a blunt needle. The area technique is no longer recommended as there is a high rate of pseudoaneurysm formation. This technique involves use of a very limited area of the access, with continued use of sharp needles and no attention to angle or direction.
Treatment
The presence or size of a pseudoaneurysm is not necessarily a reason for intervention. Treatment is typically reserved for enlarging pseudoaneurysms or changes in the overlying skin that might predispose to rupture.54 Sometimes the presence of a pseudoaneurysm can make it problematic for the dialysis technician to cannulate the access because of thrombus within the pseudoaneurysm (Fig. 77-1).
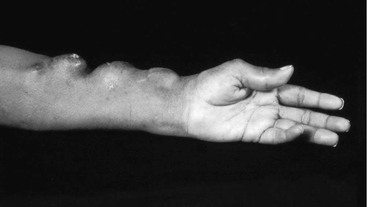
Figure 77-1 Multiple asymptomatic pseudoaneurysms occurring several years after the creation of an autogenous radial-cephalic A-V access.
Open Surgery
Traditionally, management involves either resection of the pseudoaneurysm and placement of an interposition prosthetic graft or bypass around the lesion. While the new segment is allowed to incorporate, dialysis can be continued with use of the original, intact segment. However, when multiple pseudoaneurysms are present along the length of the access, it is difficult to perform segmental replacement of the affected area; in this case, the whole length of the access needs to be replaced or bypassed. Usually, both arterial and venous ends of the aneurysmal graft can be preserved and the new prosthetic graft segment anastomosed in an end-to-end fashion to the remaining ends. While the new prosthetic segment is allowed to incorporate, the patient needs to undergo dialysis by a tunneled or nontunneled catheter. To avoid the placement of temporary catheters, the length of the access can be replaced in two separate settings. First, the segment with the larger or more ominous pseudoaneurysm is replaced. While that segment is allowed to incorporate, dialysis is performed through the original, intact segment. Then dialysis is performed through the new, incorporated segment while the second segment is replaced. The risks of performing two surgical procedures with multiple anastomoses should be discussed with the patient and compared with the risks of replacing the whole A-V access and using a catheter for continued dialysis.
Endovascular Treatment
After the introduction of covered stents, several series reported their use as an alternative to open repair.54,56–59 The advantages of endoluminal repair include the potential for immediate hemodialysis access without the need for temporary catheter placement, the capability of completing the procedure percutaneously, and the ability to identify any outflow venous stenoses that may have been responsible for the development of the pseudoaneurysm and to treat these at the time of pseudoaneurysm repair. Vesely reported that 8 of 11 patients with pseudoaneurysms treated with the Viabahn stent-graft (W.L. Gore & Associates, Inc, Flagstaff, Ariz) had concomitant venous stenosis, and 2 others required angioplasty of the venous outflow in the months preceding the pseudoaneurysm repair.57 Most pseudoaneurysms in the reported series were repaired with the Wallgraft (Boston Scientific, Natick, Mass), which Lin and associates found to allow early access without significant pseudoaneurysm formation.60 Barshes and associates reported self-expanding covered stent implantation in 18 patients with the Wallgraft, in 6 patients with the Viabahn stent-graft, and in 2 patients with the Fluency stent-graft (Bard Peripheral Vascular, Murray Hill, NJ).58 A transmitted pulse was evident in all patients for at least 24 hours, despite no angiographic evidence of an endoleak at the end of the procedure, which was similar to the observation made by Najibi and associates.54 The procedural success was 100%. Four patients developed early thrombosis, and six additional patients required subsequent interventions. The primary patency at 30 days and at 6 months by Kaplan-Meier analysis was 82% and 28%, respectively, and a total of 19 patients (73%) had complete resolution or a significant decrease in pseudoaneurysm size after endograft exclusion. In a more recent study, Shah and colleagues61 found a 100% initial success of endovascular treatment, with a 69% access patency rate at 6 months. A few patients underwent a hybrid repair with stent-graft repair of the pseudoaneurysm and operative decompression of the larger pseudoaneurysms (>4 cm). Infections of the stent-graft subsequently developed in three patients, with thrombosis developing in two. Shah and colleagues noted that pseudoaneurysm with skin erosion had a higher rate of failure because of infection (25%) than did pseudoaneurysms without erosions. The higher cost of the endovascular treatment of pseudoaneurysms remains a concern.
Autogenous Access Aneurysms
Post-stenotic, true aneurysm formation in a primary autogenous A-V access distal to the anastomosis is associated with hemodynamically significant stenosis. In the remainder of the access, the aneurysm forms within cannulation areas or along the venous outflow tract next to areas of venous damage, sometimes in association with vein junctions, valves, or rigid areas caused by prior interventions, such as venotomies or catheter insertion.
Treatment
Cannulation should not be continued with any type of autogenous access aneurysm, especially in those in which the overlying skin is attenuated.. The preferred treatment of postanastomotic aneurysms is creation of a new anastomosis with use of an uninvolved segment of vein, relocating to a more proximal anastomotic site as close to the previous anastomosis as possible. Although comparable secondary patency rates can be achieved by balloon angioplasty, surgery provides better results, with fewer inadequate dialysis sessions and a lower rate of catheter use. Partial resection and patch angioplasty of the stenotic segment with part of the resected material or a venous branch have been described. Pierce and associates reported their experience with a technique of reducing the lumen diameter to match the veins entering and exiting the aneurysm by partial resection using staples in 28 diffuse aneurysms in 12 patients.62 There was no wound infection or dehiscence and no bleeding or hematoma. The accesses were used continuously until the patients died or were lost to follow-up, with two exceptions. One access was thrombosed, and the other was ligated to relieve pain. The treatment of choice for aneurysms along the venous outflow tract is angioplasty, with selective stent placement for residual stenosis or elastic recoil; surgery is reserved for recurrent stenoses. Alternatively, “access reduction” surgery may be undertaken to preserve the site. Woo and associates described 19 patients in whom diffusely aneurysmal fistulae were managed by excision of excess vein—both length and width—with reconstruction of the vein over an 8- to 10-mm rubber catheter, with skin resection. They reported a median primary patency of 14 months, with no recurrent aneurysm formation. This involved technique does, however, require use of a tunneled catheter until the site has healed.63
Seroma
A perigraft seroma, sometimes referred to as a weeping graft, is a collection of sterile, clear, ultrafiltered serum surrounded by a nonsecretory fibrous pseudocapsule. Early seromas are not uncommon with prosthetic A-V grafts and frequently resolve without intervention. Chronic seroma is a relatively rare complication of prosthetic A-V accesses. Noninfectious fluid collections surrounding hemodialysis accesses can also represent hematoma or lymphocele. These complications are graded 0 to 3, as follows2:
Small hematomas and lymphoceles can be expected to resolve over time with simple observation; however, periprosthetic seromas usually require operative intervention. When untreated, they can become infected and cause wound dehiscence with skin necrosis, loss of A-V access puncture sites, and sometimes even loss of the access.
Etiology and Incidence
Periprosthetic seromas have been reported with both Dacron and polytetrafluoroethylene (PTFE) grafts, most commonly when they are placed in subcutaneous locations.64 Schanzer’s overview of complications found that in prosthetic A-V accesses, the seroma always occurs near the arterial anastomosis.65 The active transudation of serum-like fluid can be observed when the prosthetic material is exposed at that point. By definition, the fluid is persistent, sterile, and confined within a nonsecretory fibrous pseudomembrane.66 The exact incidence of this complication is not known; some seromas resolve spontaneously and are not reported; others are grouped with hematomas or low-grade infections that usually resolve during 1 to 2 weeks. The reported incidence of prosthetic A-V accesses placed at all locations ranges from 8 cases in 1674 PTFE grafts (0.48%)67 to 5 of 118 extra-anatomic bypasses (4.2%).68 Dauria and coworkers found significantly more perigraft seroma formation in upper arm prosthetic accesses than in the forearm location (4.5% vs 0.8%; P = .007).69
Clinical Presentation
Periprosthetic seromas generally appear within the first month after access placement, although they can occur up to several years afterward. They are generally painless but tend to enlarge over time, leading to difficulty with cannulation or, occasionally, stretching and thinning of the overlying skin, resulting in local pressure symptoms.67 The mass itself is filled with serous or gelatinous material.
Treatment
A variety of treatments have been described. Anecdotal reports cite success with the placement of microfibrillar collagen (Avitene; Bard Davol Inc, Warwick, RI) around the prosthetic graft after repeated attempts at aspiration had failed.68,70,71 Borrero and Doscher reported eight cases of periprosthetic seromas surrounding PTFE grafts.67 Their patients were treated with surgical extirpation of the entire seroma, including the portion attached to the graft. They reported success in six of the eight cases in which the original prosthetic graft was left in place. Blumenberg collected the largest series, surveying 279 cases from the 320 surgeons who responded.71 Treatment modalities included serial aspiration, observation, incision and drainage, cyst removal, and prosthetic graft replacement. Graft replacement yielded the highest cure rate at 92%. Observation alone had essentially the same success rate as aspiration (68% vs 69%); aspiration, however, led to infection or prosthetic graft thrombosis in 8%. Cyst removal, as advocated by Borrero and Doscher,67 had a 72% success rate, but infection or thrombosis occurred in 12%. Incision and drainage had only a 53% success rate, with persistence of the seroma in 40% and infection or thrombosis in 7%.66 Largely on the basis of the results of this study, the author recommended resection of the involved segment in patients with enlarging or symptomatic periprosthetic seromas. An interposition prosthetic graft composed of a different material, ideally through a new tunnel, should be used to restore continuity.65,72–75 Dauria and coworkers reported no recurrences of periprosthetic seromas in five patients treated with excision of the seroma capsule and bypass of the involved graft segment, whereas simple evacuation resulted in the loss of three of four prosthetic grafts.69 Aspiration should be reserved for cases in which there is diagnostic doubt.73 Gargiulo and associates reported two cases of proximal perigraft seromas successfully treated with percutaneous placement of Dacron-covered stents and closed suction drainage76; however, further studies are needed to clarify the role of endovascular stent-grafts for perigraft seromas.
Access-Related Hand Ischemia or Steal Syndrome
Access-related hand ischemia or the steal syndrome was first described in 1969 by Storey et al after the creation of a radial-cephalic autogenous access (i.e., Brescia-Cimino-Appel access).77 With the steal syndrome, blood flow to the hand is reduced. Steal can be a potentially limb-threatening event and must be promptly evaluated and treated if it is clinically significant. The goals for treating steal syndrome are twofold: restoration of antegrade flow sufficient to maintain distal perfusion and maintenance of the A-V access for dialysis. Steal is more common after prosthetic A-V access placement than after autogenous access creation, probably because of the larger caliber of prosthetic grafts. It typically occurs fairly rapidly after access creation but has been reported months to years later in up to 25% of cases.78,79 Steal after A-V access creation is remarkably common if one defines the syndrome as the reversal of flow in the inflow artery distal to the anastomosis. Kwun demonstrated reversal of flow in 73% of autogenous A-V accesses and in 91% of prosthetic A-V accesses.80 Duncan and coworkers, in a study of autogenous radial-cephalic accesses, demonstrated evidence of reduced blood pressure in the fingers in 80%.81 Despite the frequency of demonstrable alterations in flow, a symptomatic steal syndrome is much less common. Although some coolness in the hand and mild tingling in the fingers are reported in about 10% of new accesses, most of these symptoms resolve spontaneously after several weeks. The incidence of clinically significant steal is reportedly as low as 1% for autogenous A-V accesses placed distally in the forearm to as high as 9% for prosthetic A-V accesses based on the brachial artery.73,82–85 Steal can be graded 0 to 3, as follows2:
Grade 1: Mild—cool extremity, few symptoms, flow augmentation with access occlusion
Grade 2: Moderate—intermittent ischemia only during dialysis, claudication
Intervention is not necessary for grades 0 and 1. Intervention is sometimes necessary for grade 2 and mandatory for grade 3.
Prevention
Before A-V access creation, arterial evaluation with bilateral upper extremity blood pressures and an Allen test are mandatory. The Allen test is performed by elevating the arm with the fist clenched. Both the radial and ulnar arteries are compressed and then released one at a time to observe for the flush of reperfusion to the hand. The test is repeated, releasing the other artery first. Results can be difficult to interpret in those with significant anemia or dark skin pigmentation. Significantly calcified vessels, which are often seen in diabetic patients, can also make compression difficult. A more sophisticated study involves performing the evaluation with a handheld continuous wave Doppler probe; triphasic flow that augments with collateral compression is a normal finding, and no further evaluation is necessary.86 If patients are found to have an incomplete palmar arch with radial artery dominance or blood pressure differentials greater than 20 mm Hg,78 that extremity or artery should not be used for access creation unless the underlying problem can be corrected first owing to the risk of ischemia. Arterial duplex ultrasound may be necessary in patients with questionable Allen test results or in those with any concern for upper extremity ischemia. Angiography is indicated for assessing patients with abnormal arterial examination findings or discrepant blood pressures to identify proximal stenoses that may be amenable to intervention, thereby permitting use of the arm for future access. A history of symptoms for upper extremity ischemia should be investigated before access creation. The digital-brachial index (DBI) has been suggested by Goff and associates as a potential method of identifying patients at increased risk for steal.87 Digital pressure is obtained in the third digit with digital pneumatic cuffs and compared with the ipsilateral brachial pressure. DBIs have been used to identify patients at risk.87,88 However, preoperative and intraoperative DBI measurements have not been useful in predicting which patients will actually develop clinically significant steal owing to the lack of a reliable threshold below which steal is inevitable.87,89
Although no preoperative test can reliably predict who will develop steal, it is especially important to determine which patients are at increased risk. These include elderly patients, patients with multiple prior access procedures, patients with a history of significant peripheral arterial occlusive disease or vascular surgery, patients with a prior history of steal, and patients with diabetes, all of whom are likely to have underlying upper extremity atherosclerosis.87–91 Use of a prosthetic A-V access and use of the brachial artery (rather than a more distal artery as the donor vessel) also may predispose a patient to steal.92–94 Recent recommendations have encouraged use of the proximal radial artery, when feasible, over the brachial artery, especially in high-risk patients.95 Berman and associates showed that only 3 of 884 patients (0.3%) who had otherwise normal findings on preoperative arterial examination could have been identified as being at risk for ischemia by segmental or digital pressure evaluation before surgery.78 They concluded that this low yield does not justify routine testing before access placement.
Pathophysiology
The creation of an A-V access normally produces an alteration in blood flow patterns or a “physiologic” steal phenomenon96 that is more common or more pronounced in upper arm A-V accesses than in forearm configurations.93 Physiologic steal results from the lower resistance in the venous system, which then may induce a reversal of flow in a portion of the arterial system. Most inflow arteries are sufficiently large to meet the increased blood flow demand, given appropriate increases in the heart rate and cardiac output and, potentially, arterial vasodilatation. However, diseased arteries are not always able to compensate with vasodilatation. When this compensation is inadequate, symptomatic steal develops. On the basis of physiologic models, steal is more likely to occur when there is increased vascular resistance peripheral to the fistula, inflow stenosis, or increased access size leading to very high flow rates.97 The development of symptoms during dialysis may be due to a reduction in systemic blood pressure rather than to an increased shunting of brachial blood flow. The role of increased peripheral resistance may explain why diabetic and hypertensive patients are at increased risk for the development of steal. Wixon and colleagues likened the hemodynamics to a Wheatstone bridge, which also explains the improvement in symptoms over time with the development of collaterals around the prosthetic or autogenous access.93 The flow rate, high or low, through the access has also been used by many to categorize the steal and to suggest alternative forms of therapy to address the steal.
Incidence
With the aging of the hemodialysis population and the increase in arterial disease caused by diabetes and hypertension, the incidence of symptomatic peripheral ischemia to the hand or arm (pain, necrosis in one or more fingertips) is increasing, but it is still uncommon (1% to 4%).84 Milder symptoms may occur in up to 10% of cases and improve during weeks to months.92 A decrease in distal perfusion pressures is found regularly and is more pronounced in patients with advanced anteromedial sclerosis. In such patients, ischemic symptoms seem less dependent on access flow volume than on the degree of peripheral arterial obstructive disease.
Diagnosis
Clinical Presentation
Deciding which patients need intervention for steal syndrome requires clinical judgment. Most patients with grade 1 steal may be managed expectantly. Many patients experience transient mild symptoms, such as coolness of the hand, numbness and paresthesia of one or more fingers, or pain and stiffness of the fingers.83,86 These mild symptoms can be watched with the expectation that most will resolve within a few weeks or at least not progress. However, patients should return for follow-up on a frequent basis because worsening of symptoms can occur quickly and may result in permanent injury, such as muscle atrophy. Patients with grade 2 steal, pain with use or during hemodialysis, are at greater risk for complication and need to be closely monitored to determine whether intervention is necessary. Most reports show that between one half and two thirds of patients who develop steal do so within the first 30 days.78,79 Grade 3 symptoms, rest pain or motor impairment immediately after surgery, requires immediate reoperation.88,98–100 Other severe symptoms of ischemia, including progressive numbness or pain, pallor of the hand, diminished sensation, ischemic ulcers, progressive dry gangrene, and atrophy of the hand muscle, demand urgent intervention.83 Typically, early symptoms occur with gradual tissue loss; however, if these are ignored, there is a rapid final deterioration leading to necrosis and gangrene of the digits. It is important to differentiate the findings of hand ischemia from those of carpal tunnel syndrome, tissue acidosis, and edema from venous hypertension and monomelic ischemic neuropathy (discussed later in the chapter). However, ischemia should be the presumed diagnosis until proven otherwise.98 Symptoms of true steal syndrome are frequently exacerbated during dialysis. If steal syndrome is suspected, urgent and thorough vascular evaluation is necessary. Ischemic complications can also be seen in lower extremity A-V accesses. Autogenous A-V accesses in this location were found to have a higher incidence of ischemia than prosthetic accesses in a meta-analysis by Antoniou et al (20.97% vs 7.18%; P < .05),38 with resultant amputation rates of up to 7%. This was found to be worse in elderly diabetic patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


