Chapter 74
Hemodialysis Access
Dialysis Catheters
Karen Woo, Vincent L. Rowe
Central venous catheters for hemodialysis are categorized by their intended duration as either acute or chronic. Chronic hemodialysis catheters are placed in a tunneled fashion and have a subcutaneous cuff at the skin exit site for tissue ingrowth. Acute catheters do not have a cuff and are placed percutaneously without a tunnel. As a result, acute catheters are at higher risk for infectious complications and can easily become dislodged. Thus, acute catheters should be placed only in hospitalized patients and used for a short time.1
This chapter focuses on tunneled hemodialysis catheters and their management, but the potential complications related to placement of acute hemodialysis catheters mirror those of tunneled hemodialysis catheters. Because of the short duration of use of acute catheters, long-term complications are less frequent but infectious complications are more common.
Indications
The tunneled hemodialysis catheter serves a valuable role in the treatment of patients with end-stage renal disease and has distinct advantages over autogenous (arteriovenous fistula [AVF]) and prosthetic (arteriovenous graft [AVG]) arteriovenous hemodialysis access. The benefits of tunneled hemodialysis catheters include immediate use for hemodialysis, uncomplicated and needle-free connection to the dialysis circuit, elimination of cannulation site complications, and simple insertion technique that can be performed by many different interventional specialists.2
The most common indication for placement of a tunneled hemodialysis catheter is for urgent hemodialysis while the patient is waiting for an AVF to be created or to mature. Hemodialysis through a mature AVF represents the most optimal form of hemodialysis access, and ideally patients should be referred for placement of permanent access long before the need for hemodialysis. In this way, patients have an adequate amount of time for AVF maturation, and the requirement for a tunneled hemodialysis catheter is eliminated altogether. Unfortunately, demise of renal function is not a linear estimation. At the same time, care before end-stage renal disease is often inadequate, resulting in late referral to a nephrologist and to an access surgeon.
Other indications include patients in whom an AVF or AVG is not anatomically feasible or who are not operative candidates because of advanced comorbidities. Temporary hemoaccess is also indicated after revision of a permanent hemodialysis access for management of a complication (e.g., access revision for pseudoaneurysm formation or infection), after placement of a peritoneal dialysis catheter, and for a chronic ambulatory peritoneal dialysis patient requiring abdominal or inguinal surgery.
Types of Catheters
The feasibility of using a felt cuffed catheter for prolonged hemodialysis was first described in 1988.3 The authors documented a median catheter survival of 8 weeks for a tunneled hemodialysis catheter placed in the subclavian vein. Since this initial report, catheter design and preferred anatomic position for placement have evolved. Early cuffed catheters were straight in configuration and stiff. These have been largely supplanted by precurved flexible catheters with various tip designs.
Design Characteristics
Catheter designs aim to achieve one main goal: adequate dialysis clearance at a relatively high flow rate. Currently used hemodialysis machines maintain flow rates of 300 to 350 mL/min through indwelling catheters to provide reasonable clearance times for the patients.4 To support the high-flow demand, some manufacturers have increased the catheter lumen size up to 16F. In addition, the phenomenon of recirculation must be minimized to ensure adequate clearance. The “arterial lumen” of the catheter is the outflow to the dialysis machine from the patient; the “venous lumen” is defined as the inflow from the machine back to the patient. Access recirculation is the reentry of dialyzed blood from the venous lumen directly into the arterial lumen, thus bypassing the systemic circulation and leading to inefficient dialysis.5
Design Categories
Numerous manufacturer modifications exist in an effort to satisfy the requirements of high flow rates and minimal recirculation. The modifications fall into four general categories.
Split Tip.
Split-tip catheters have a double-lumen, single-body configuration in the midbody but separate into two distinct distal tips, each with side holes in all directions (Fig. 74-1A).
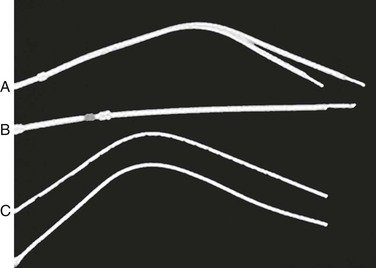
Figure 74-1 Three types of tunneled hemodialysis catheters: split-tip catheter (A), staggered-tip or step-tip catheter (B), and dual catheter (C). (From Richard HM, 3rd, et al: A randomized, prospective evaluation of the Tesio, Ash split, and Opti-flow hemodialysis catheters. J Vasc Interv Radiol 12:431-435, 2001.)
Step Tip.
The staggered-tip or step-tip catheter is a double-lumen, single-body catheter with the venous limb extending at least 2.5 cm beyond the inflow tip (Fig. 74-1B).
Dual Catheter.
The dual catheter design consists of two completely separate catheters that can be inserted in two different locations (Fig. 74-1C).
Symmetric Tip.
The Tal Palindrome (Covidien, Mansfield, Mass) is the only catheter that has a symmetric tip design with equal length of arterial and venous limbs and biased spiral ports. The design of the ports allows inflow to occur through the most proximal portion of the port and outflow to occur as a jet directed away from the catheter tip (Fig. 74-2).6
Shape and Material
Because of the complication of catheter-associated central vein stenosis, the anatomic preference for catheter placement migrated cephalad from the subclavian veins to the internal jugular veins.1 During the same time, straight catheter designs evolved to adopt a premade curve to avoid kinking of the catheter at the vein entry site in the neck. The material composition of the tunneled hemodialysis catheter also affects flow rates. Silicone rubber and Silastic catheters are softer and more pliable, increasing patient comfort when the catheter is inserted into the internal jugular vein and tunneled over the clavicle. Newer polyurethane catheters have a stiffer construct and higher tensile strength and thereby allow thinner walled catheters with smaller outer diameters. Numerous reviews directly compare performance of individual tunneled hemodialysis catheters with specific outcome variables of flow rate, recirculation time, and patency. Unfortunately, despite isolated beneficial characteristics of a particular catheter, no universal significant benefit has been demonstrated by one catheter over the competitors.2,4,5,7
Preoperative Evaluation
History and Physical Examination
As with any clinical assessment, the proper preoperative evaluation of a patient begins with a detailed history and physical examination. Specific inquiries should include details such as prior long-term central line placement, prior AVF or AVG placement, prior tunneled hemodialysis catheter infections, history of a coagulation disorder, and history of a pacemaker. Physical examination of the neck and chest is critical in the evaluation of the hemodialysis patient. Evidence of a previously placed tunneled hemodialysis catheter, previous permanent accesses, upper extremity or facial edema, and ipsilateral venous distention with visible venous collaterals should alert the clinician to the possibility of central veno-occlusive disease.
Numerous specialists have acquired the skill set to properly place a tunneled hemodialysis catheter. However, there is a significant advantage in terms of continuity of care when the same practitioner places the tunneled hemodialysis catheter and the permanent access or when a team approach coordinates these procedures. There are a finite number of access sites available for both tunneled catheter and permanent access placement. Therefore, a comprehensive plan that embraces all forms of hemodialysis access may provide maximum benefit of each crucial access site.
Central Venous Imaging
Color-Flow Venous Duplex Imaging
Similar to the screening of hemodialysis patients for permanent dialysis access, noninvasive color-flow duplex imaging is the first-line method of preoperative imaging for the tunneled hemodialysis catheter. Patency of internal jugular veins and axillary veins is easily identified with compression of the vein. However, as imaging moves toward the central chest, the air interface with the lung tissue as well as obstructing bone structures makes central vein imaging virtually impossible, limiting color-flow venous duplex imaging mainly to the vein entry site.
Magnetic Resonance Venography
Contrast-enhanced magnetic resonance venography (MRV) is a noninvasive modality for imaging of the central veins. Three-dimensional gadolinium-enhanced MRV has been evaluated in the assessment of central venous steno-occlusive disease specifically in hemodialysis patients. In a series of 14 patients, three-dimensional gadolinium-enhanced MRV had a 93% sensitivity in identifying central vein occlusions and stenoses greater than 50% when it was directly compared with digital subtraction angiography.8 Despite the accuracy of MRV, gadolinium must be administered with caution in patients with a glomerular filtration rate of less than 30 mL/min because of the risk of gadolinium-induced nephrogenic systemic fibrosis. Although nephrogenic systemic fibrosis is rare even in this patient population, the condition has not been described in patients with normal renal function.
Computed Tomographic Venography
Computed tomographic venography (CTV) is similar to MRV in being able to image multiple vessels in the chest in one setting. However, CTV does have the advantages of being readily available in most medical centers, fast acquisition times, and fewer deleterious contrast agent concerns. In medical centers dedicated to use of this imaging modality for the evaluation of hemodialysis patients, anecdotal evidence points to an acceptable level of accuracy for the evaluation of central venous disease. A small study of 18 patients was performed to compare CTV and digital subtraction venography for the diagnosis of benign thoracic central venous obstruction. The results of the study demonstrated that CTV findings correlated closely with those of digital subtraction venography.9
Catheter-Based Contrast Venography
Catheter-based contrast venography remains the “gold standard” for diagnosis of central vein stenosis or occlusion. Contrast venography has the distinct advantage of allowing the clinician to initiate endovascular treatment if a significant stenosis is detected at the time of venography. In addition, it is often possible to perform catheter-based venography with a much smaller volume of contrast material than what is required for CTV, reducing the risk of nephrotoxicity.
Catheter Insertion
Site Selection
Numerous venous access sites exist for insertion of a tunneled hemodialysis catheter. However, the right internal jugular vein is the preferred access site because it has the best patency, presumably owing to less kinking. In a prospective evaluation, factors affecting long-term survival of tunneled hemodialysis catheters were analyzed in a cohort of 812 catheters in 492 patients.2 Factors that were associated with immediate failure of the tunneled hemodialysis catheter were malposition and kinking. A tunneled hemodialysis catheter placed in the right internal jugular vein demonstrated significantly longer survival compared with one placed in the left internal jugular vein. Tunneled hemodialysis catheters placed in the femoral vein had the worst long-term survival. The subclavian vein should be avoided if possible to prevent catheter-induced subclavian stenosis, which would negatively affect the future placement of ipsilateral permanent access.2,4
A tunneled hemodialysis catheter from the right internal jugular vein should be 17 to 19 cm in length; catheters from the left are slightly longer. When femoral access is used, long catheters (24 to 31 cm) should be placed so that the tip can reach into the inferior vena cava to produce the best flow rates.
Technique
Tunneled hemodialysis catheter insertion should be performed in a procedure room under fluoroscopic guidance. Bedside placement is discouraged because of the need for proper sterility, the possibility of additional endovascular procedures (such as venography and venoplasty), and the requirement for fluoroscopy. The procedure is usually performed under local anesthesia with conscious sedation. Patients who have had numerous prior catheters have an increased risk of central venous stenoses or occlusions that may increase the complexity of the procedure. In such cases, general anesthesia is preferred. Preoperative antibiotic prophylaxis directed at gram-positive bacterial strains is administered before skin incision in each case. The skin is then prepared with isopropyl alcohol and chlorhexidine solutions.
Ultrasound evaluation with a sterile covered transducer confirms access vein patency. The site of vein cannulation should be 3 to 4 cm cephalad to the clavicle. Real-time ultrasound guidance is used to access the vein. The puncture is performed with a micropuncture 21-gauge needle, 0.018-inch guide wire, and 5F coaxial catheter (Fig. 74-3). After successful vein cannulation with the micropuncture needle, the introducer and 0.018-inch wire are removed and exchanged for a 0.035-inch wire. In preparation for the large-bore hemodialysis catheter, a 1-cm skin incision is made surrounding the wire entry site (Fig. 74-4, short arrow). Fluoroscopic imaging should be used during all wire maneuvers to confirm wire position.
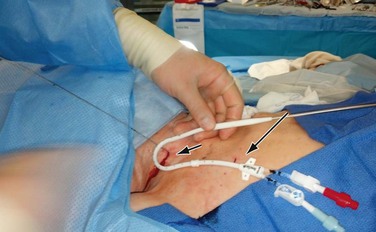
Figure 74-4 Placement of the tunneled hemodialysis catheter alongside the anticipated catheter course to determine where to position the exit site. Internal jugular vein puncture site (short arrow); upper chest catheter exit site (long arrow).
Antegrade Placement
A small incision is made on the anterior chest where the tunneled hemodialysis catheter is to exit the subcutaneous tunnel (Fig. 74-4, long arrow). The exit site of the tunneled hemodialysis catheter should be inferior and lateral to the vein entry site in location. The exit site should also be positioned such that the felt cuff on the catheter shaft is 1 cm proximal to the exit site. Placement of the tunneled hemodialysis catheter alongside the anticipated catheter course is useful in determining where the exit site should be positioned. The tunneled hemodialysis catheter is then attached to the tunneling device and passed subcutaneously from the chest incision up through the vein entry site incision in the neck, traversing anterior to the clavicle en route (Fig. 74-5). The track of the wire is sequentially dilated, and the final introducer and peel-away sheath unit is inserted until the sheath is “hubbed” at the skin (Fig. 74-6). The introducer and wire are removed, and the tunneled hemodialysis catheter is inserted through the peel-away sheath (Fig. 74-7). Once the tunneled hemodialysis catheter is fully inserted, the peel-away sheath is withdrawn.
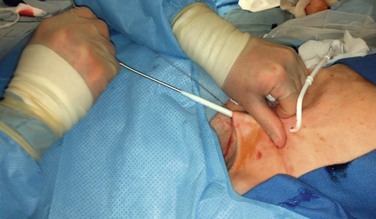
Figure 74-5 Tunneling of the catheter subcutaneously from the chest incision to the neck incision anterior to the clavicle.
Each lumen of the catheter is aspirated and flushed with dilute heparin (100 units of heparin sulfate per milliliter of normal saline). Both of these actions should be easily accomplished without resistance. If resistance is encountered, the tunneled hemodialysis catheter should be examined under fluoroscopy to rule out a kink in the path of the catheter or malpositioning of the tip. Ideally, the most distal tip of the catheter should be in the distal superior vena cava/proximal right atrium border. This preferred position corresponds to the shadow of the right mainstem bronchus (Fig. 74-8).
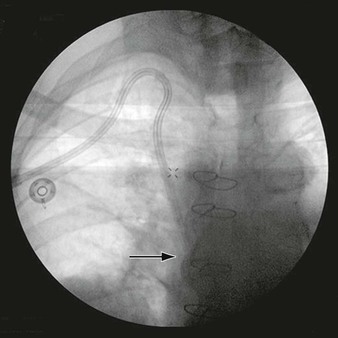
Figure 74-8 Chest radiograph demonstrating proper position of the tip of the tunneled hemodialysis catheter (arrow) at the level of the right mainstem bronchus.
The chest wall and vein entry site incisions are closed with absorbable sutures, and the tunneled hemodialysis catheter is secured to the chest wall with nonabsorbable sutures. Each lumen of the tunneled hemodialysis catheter is filled with the manufacturer-indicated volume of concentrated heparinized saline (1000 units/mL) to protect against intracatheter thrombosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree