CHAPTER 2
Heart Failure Presentations and Functional Types
—Samuel Levine, MD, from Clinical Heart Disease, 19511
Heart Failure Designation Based on Left Ventricular Systolic Function
The four stages of heart failure are based on the progression of structural heart disease and development of symptoms. Identifying the presence or absence of systolic dysfunction is another important distinction.
LEFT VENTRICULAR EJECTION FRACTION IN HEART FAILURE
Heart failure occurs with either a reduced (≤ 40%) or preserved (> 40%) left ventricular ejection fraction (EF). Individual center,2 multicenter registry,3 and randomized trial data,4 demonstrate bimodal distributions in left ventricular ejection fraction with nadirs between 40% and 50% (Figure 2.1). The demographics and clinical etiology of patients with reduced (HF-rEF) or preserved (HF-pEF) ejection fraction also differ (Table 2.1). Nevertheless, significant overlap of these 2 groups exists.5 The 2013 ACCF/AHA guidelines define patients with EF between 41% and 49% as borderline HF-pEF with clinical findings similar to those with EF ≥ 50%.6
Published cutoff values for HF-rEF versus HF-pEF range from 40% to 55%.6–8 In this book, HF-rEF will be defined as an ejection fraction of less than or equal to 40% because of the common use of this value for entry into trials of therapies found effective in patients with systolic dysfunction.
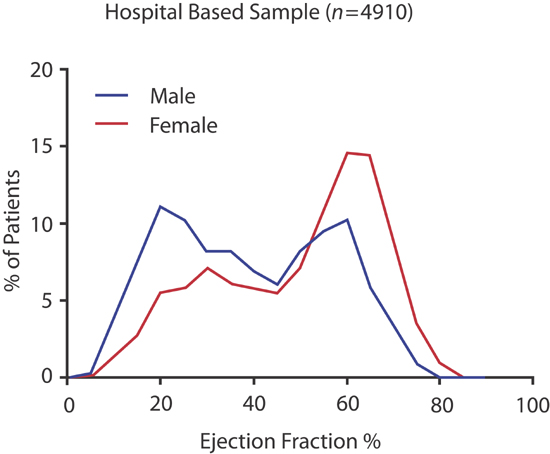
FIGURE 2.1 Bimodal distributions in left ventricular ejection fraction in patients admitted with heart failure at Mayo Clinic ( n = 4910).2 Source: Adapted from Borlaug & Redfield, Circulation. 2011;123(18):2006-2013; discussion 2014, with permission.
TABLE 2.1 Hospitalized heart failure patient demographics with EF < 50% or ≥ 50% (Mean ± SD).9
CHARACTERISTICS | EJECTION FRACTION | EJECTION FRACTION | P VALUE |
Age (yr) | 71.7 ± 12.1 | 74.4 ± 14.4 | < 0.001 |
Male sex (% of patients) | 65.4 | 44.3 | < 0.001 |
Body-mass index (kg/m2) | 28.6 ± 7.0 | 29.7 ± 7.8 | 0.002 |
Serum creatinine on admission (mg/dL) | 1.6 ± 1.0 | 1.6 ± 1.1 | 0.31 |
Hemoglobin on admission (g/dL) | 12.5 ± 2.0 | 11.8 ± 2.1 | < 0.001 |
Hypertension (% of patients) | 48.0 | 62.7 | < 0.001 |
Coronary artery disease (% of patients) | 63.7 | 52.9 | < 0.001 |
Atrial fibrillation (% of patients) | 28.5 | 41.3 | < 0.001 |
Diabetes (% of patients) | 34.3 | 33.1 | 0.42 |
Substantial valve disease (% of patients) | 6.5 | 2.6 | < 0.001 |
Ejection fraction (%) | 29 ± 10 | 61 ± 7 | < 0.001 |
Source: Adapted from Owan et al., N Engl J Med. 2006;355(3):251-259, with permission.
PRESERVATION OF RESTING STROKE VOLUME
Until the late stages of heart failure, resting stroke volume is usually preserved in both HF-rEF and HF-pEF. In a patient with HF-rEF, a decreased ejection fraction is associated with an increased end-diastolic volume. With HF-pEF, a noncompliant left ventricle is associated with an increased diastolic filling pressure to maintain end-diastolic volume.
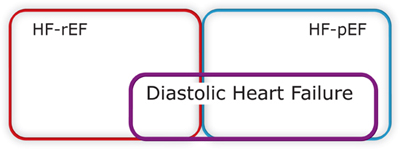
ROLE OF DIASTOLIC DYSFUNCTION IN HF-pEF
Doppler echocardiographic characterization of patterns of blood or tissue velocities during ventricular filling has been used to define abnormal filling patterns, diastolic dysfunction, and estimation of left atrial pressure (see Chapter 7). Diastolic dysfunction, however, is not equivalent to HF-pEF. Approximately one-third of patients with HF-pEF do not have identifiable diastolic dysfunction by echocardiography.10 In addition, patients with a reduced ejection fraction may also have diastolic abnormalities, highlighting the linked relationship between the hemodynamics of systole and diastole.11
Finally, some individuals may have the echocardiographic findings of diastolic dysfunction without any past or present clinical syndrome of heart failure.10
Therefore, in the presence of a left ventricular ejection fraction greater than 40%, the diagnosis of HF-pEF depends on the clinical findings consistent with an elevated left ventricular filling pressure (e.g., pulmonary edema) supported by echocardiographic findings or catheter pressure measurements. The HF-pEF patient typically will have elevated blood levels of brain natriuretic peptide (BNP) (see the Biomarkers section below, and Chapter 5), although not generally as high as in HF-rEF.12
WHEN TO TREAT HF-pEF LIKE HF-rEF
Two subgroups of patients with HF-pEF should be considered for pharmacologic therapies validated for HF-rEF. The first are those with a borderline EF of 40% to 50% with clinical features of either HF-rEF or HF-pEF. The second subgroup includes patients who previously had an EF ≤ 40% who now have recovered to a higher EF. Depending on the etiology of the previous reduced EF, this subgroup may be prone to relapses of reduced EF and therefore will benefit from long-term preventive therapy.13
Acute and Chronic Presentations of Heart Failure
In addition to the treatment of symptoms and restoring hemodynamic stability, different presentations of heart failure have potentially different therapeutic goals.
NEW-ONSET HEART FAILURE
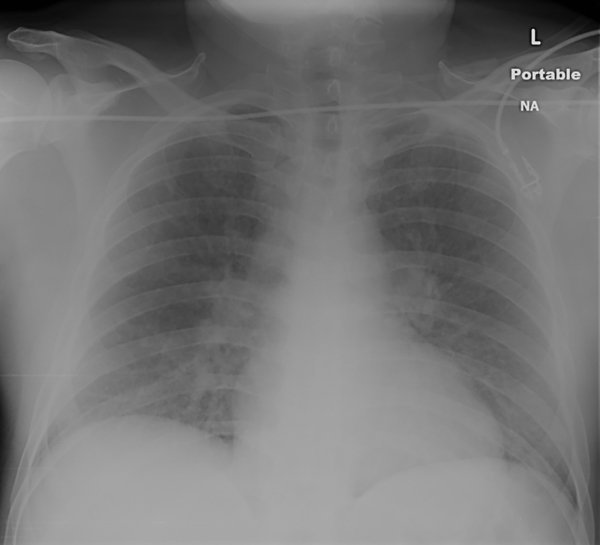
If new in onset, then determining the etiology of heart failure is a priority. In the presence of acute pulmonary edema (Figure 2.2), an aggressive approach is necessary, and improvement of symptoms will follow amelioration of abnormal hemodynamics. In heart failure with acute ischemic syndromes, revascularization improves outcomes.14,15
FIGURE 2.2 New-onset heart failure. This chest x-ray shows a 49-year-old man with acute anterior myocardial infarct and new-onset heart failure. Pulmonary congestion is present with a relatively normal-sized cardiac silhouette. Urgent percutaneous coronary revascularization and diuretics led to improvement.
COMPENSATED CHRONIC HEART FAILURE
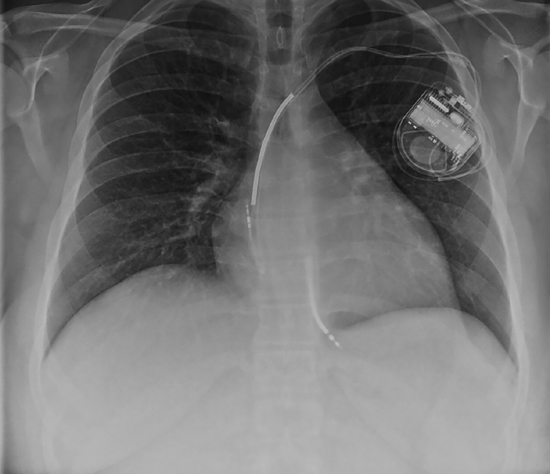
In a patient with compensated heart failure, there are few active symptoms by definition; after treating causes of cardiac dysfunction, the priority may be to delay or reverse progression of structural heart disease by blunting neurohumoral activation. These patients may have either newly recognized or known chronic left ventricular dysfunction without overt resting findings (Figure 2.3). Symptoms, however, may appear with activity.
FIGURE 2.3 Compensated chronic nonischemic cardiomyopathy. In this 24-year-old man, heart size is increased, but lung fields are clear. An implantable cardioverter-defibrillator (ICD) device is present with leads in the right atrium and right ventricle.
ACUTE-ON-CHRONIC HEART FAILURE
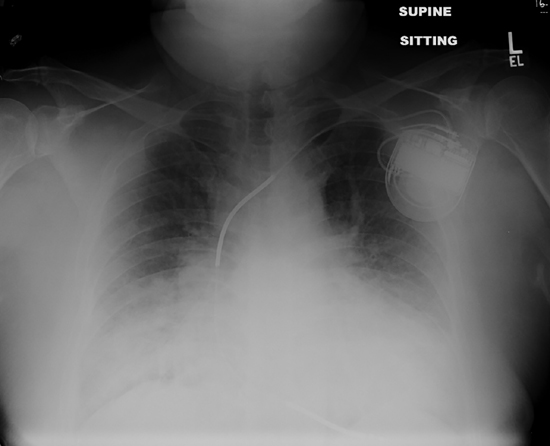
In a patient with a history of heart failure who presents in a decompensated state, the priority is less to make a diagnosis of the etiology of heart failure (this is usually known) and more to identify the precipitating factors for the decompensation (see Chapter 9). A patient may have pulmonary edema, systemic congestion, or both (Figures 2.4 and 2.5). Findings of low cardiac output, including fatigue, poor mentation, hypotension, and hepatic and renal dysfunction, may coexist.
FIGURE 2.4 Acute-on-chronic heart failure with reduced EF. The chest x-ray shows a 60-year-old man with history of ischemic cardiomyopathy, moderate renal insufficiency, and increasing shortness of breath. Marked cardiomegaly is obscured by pulmonary congestion and bilateral pleural effusions.
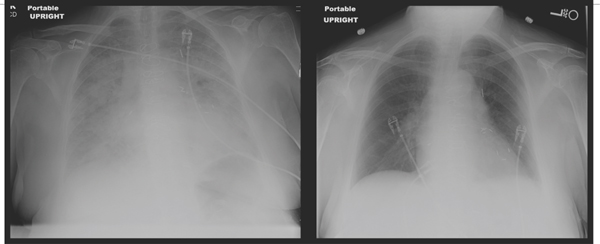
FIGURE 2.5 Acute-on-chronic heart failure with preserved EF. This x-ray shows a 79-year-old female presenting with acute shortness of breath and atrial fibrillation with rapid ventricular response associated with history of previous HF-pEF, coronary artery bypass surgery, hypertension, and diabetes. Left ventricular ejection fraction was 70%. Diuresis and slowing of heart rate led to resolution of pulmonary edema in 3 days.
Common Causes of Heart Failure
Hemodynamic features of heart failure with circulatory congestion or inadequate tissue perfusion may be similar regardless of the etiology of heart pump insufficiency. HF-rEF with systolic dysfunction usually predominates following myocardial infarction, chronic volume overload, or dilated cardiomyopathy. HF-pEF with diastolic dysfunction typically develops with hypertension and diastolic dysfunction, myocardial ischemia without infarction, aortic stenosis, and with hypertrophic or restrictive cardiomyopathies (Figure 2.6).16
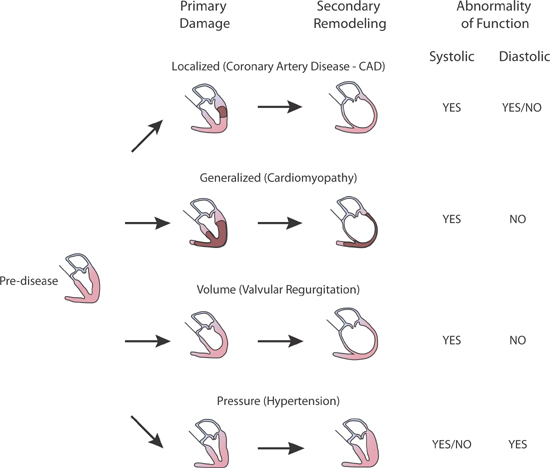
FIGURE 2.6 Secondary remodeling and heart failure. Examples of types of heart failure with a primary insult progressing via secondary remodeling.16 Source: Reproduced with permission from Gorlin R., Circulation. 1987;75(suppl IV):108-111.
HEART FAILURE CAUSED BY CORONARY ARTERY DISEASE AND MYOCARDIAL INFARCTION
In patients with recent or remote myocardial infarctions, the severity of the associated heart failure correlates with the extent of left ventricular muscle volume replaced by necrosis or fibrosis.17 In a meta-analysis of population-based versus clinical trial studies, the mean in-hospital incidence of heart failure after acute myocardial infarction was 37% in population-based studies compared to 18% in clinical trials, likely reflecting the exclusion of some high-risk patients in clinical trials.18 Compared to those with uncomplicated myocardial infarction, patients with myocardial infarction and heart failure had up to a five-fold increase in 1-year mortality.18 In a multicenter trial of reperfusion for acute ST-elevation myocardial infarction, the profound heart failure state of cardiogenic shock was present in less than 1% of patients at the time of admission, but developed in 6.4% during hospital admission.19 The later development of cardiac pump failure may be mediated by time-dependent neurohumoral activation, fluid retention, myocardial remodeling, or reinfarction.20
< div class='tao-gold-member'>