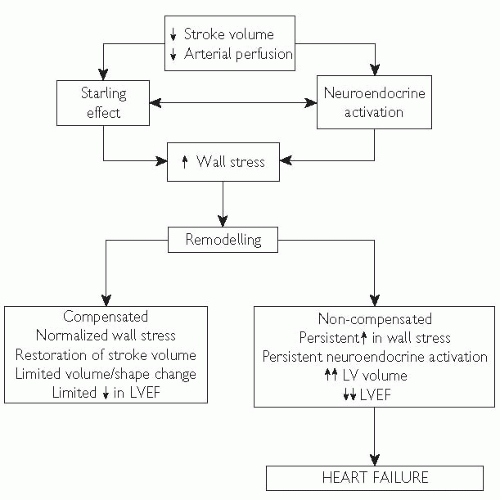
Acute pulmonary oedema: assessment, p. 724.
Acute pulmonary oedema: assessment, p. 724.Aetiology of heart failure | ||
|---|---|---|
|
Inappropriate reduction in therapy: self-discontinuation or iatrogenic withdrawal of diuretics, angiotensin-converting enzyme inhibitor (ACE-I), digoxin, as well as dietary excess of salt are recognized precipitants. Education of the patient/family is important.
Cardiac arrhythmias: most commonly atrial fibrillation (AF), but any tachyarrhythmia will further reduce LV filling and stroke volume, and may exacerbate ischaemia. Marked bradycardia reduces cardiac output, especially if stroke volume cannot increase any further.
Myocardial ischaemia or infarction: exacerbates LV dysfunction, and may worsen mitral regurgitation (MR) due to ischaemia of papillary muscles.
Infection: respiratory infections are more common, but any systemic sepsis can precipitate heart failure due to a combination of factors such as direct myocardial depression from inflammatory cytokines, sinus tachycardia, fever, etc.
Anaemia: this causes a high-output state that may precipitate acute heart failure, and may exacerbate underlying ischaemia.
Concomitant drug therapy: drugs that directly depress myocardial function (e.g. calcium antagonists—verapamil, diltiazem; many antiarrhythmics, anaesthetics, over-enthusiastic initiation of β-blockers, etc.), as well as drugs causing salt and water retention (e.g. non-steroidal antiinflammatory drugs (NSAIDs), oestrogens, steroids, COX-2 antagonists) may precipitate heart failure.
Alcohol: this is directly toxic and, in excess, can depress myocardial function as well as predispose to arrhythmias.
Pulmonary embolism: the risk increases in the immobile patient with low-output state and AF.
Table 7.1 Population-attributable risk of heart failure related to various risk factorsa | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Conditions mimicking heart failure | ||
|---|---|---|
|
|
gastrointestinal symptoms secondary to congestive hepatomegaly, ascites, reduced bowel perfusion, and oedema (abdominal distension and pain, anorexia, bloating, nausea, constipation, jaundice)
genitourinary symptoms secondary to impaired renal perfusion (oliguria/anuria, urinary frequency, nocturia)
cerebrovascular symptoms secondary to cerebral hypoperfusion and associated electrolyte abnormalities (confusion, memory impairment, anxiety, headaches, insomnia, bad dreams or nightmares, psychosis with disorientation, delirium, or hallucinations)
musculoskeletal symptoms (gout, carpal tunnel syndrome, muscle cramps).
NYHA classification of heart failure | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
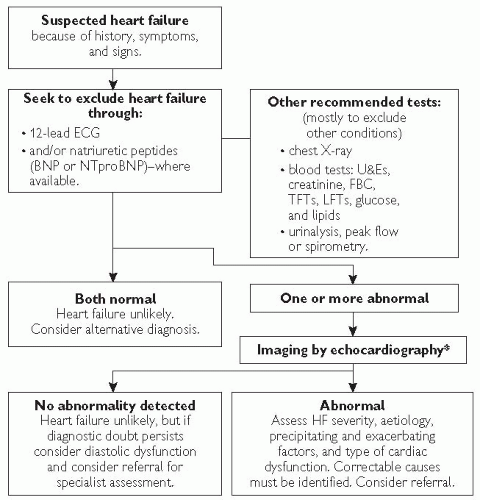 Fig. 7.2 Algorithm summarizing recommendations for the diagnosis of heart failure. *Alternative methods of imaging the heart should be considered when a poor image is produced by transthoracic Doppler 2D-echocardiography—alternatives include transoesophageal echocardiography (TOE), radionuclide imaging, or cardiac magnetic resonance imaging (MRI). |
ECG: although there are no specific changes in heart failure, a normal ECG is observed in only 2% of cases and should encourage the clinician to consider an alternative diagnosis in the absence of firm clinical signs. Common findings include: sinus tachycardia/bradycardia, arrhythmias, voltage criteria for left ventricular hypertrophy (LVH), evidence of current or past ischaemia/infarction, and conduction system defects.
Chest X-ray (CXR): permits assessment of pulmonary congestion and may demonstrate other non-cardiac causes of dyspnoea. Common findings include: cardiomegaly, pulmonary congestion with alveolar oedema, prominent upper lobe vessels, ‘bat’s wings’ and Kerley B lines and pleural effusions (see Fig. 7.3).
Echocardiography (ECHO): this is the key investigation in patients with HF and is mandatory for confirming the diagnosis. Apart from documenting systolic and diastolic left ventricular function, the scan is useful in the identification of various causes or complications of heart failure (see Table 7.3). If an echocardiogram does not confirm a diagnosis of HF despite suggestive clinical symptoms and signs, consider an alternative diagnosis or a referral for a specialist review.
Natriuretic peptides: evidence exists supporting the use of plasma concentrations of natriuretic peptides for diagnosing, staging, or even identifying patients at risk for clinical events. A normal plasma concentration in an untreated patient has a high negative predictive value, making HF an unlikely cause of symptoms.
Blood tests: FBC, U&Es, LFTs, TFTs, glucose, uric acid.
Blood tests: troponin I or T, iron studies, folate, vitamin B12, autoimmune screen, immunoglobulins and protein electrophresis, serum ACE
Viral titres
Urine sample: albumin/creatinine ratio, 24-hour urine collection for protein, catecholamines, Bence-Jones protein
Arterial blood gases
Pulmonary function tests
Exercise testing
Ambulatory ECG monitoring (QT dispersion, heart-rate variability)
Stress imaging
Radionucleotide ventriculography
Cardiac magnetic resonance
Coronary angiography (computed tomography (CT) or conventional)
Myocardial biopsy
Right-heart catheterization.
Table 7.3 Conditions predisposing to or complicating heart failure that may be identified by echocardiography | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
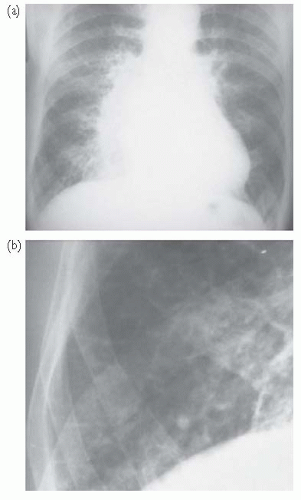 Fig. 7.3 CXR findings in heart failure. (a) There is cardiomegaly with prominent upper lobe vessels and alveolar oedema (‘Bat’s wing shadowing’); (b) magnification of the right costophrenic angle showing septal lines (Kerley B lines) due to interstitial oedema. |
Establish a firm diagnosis of HF
Attempt to determine the aetiology and ascertain the severity of HF
Correct precipitating or exacerbating factors
Multidisciplinary approach to treatment (HF is a complex syndrome necessitating the involvement of a number of healthcare professionals in the community and secondary care including: general practiitoner (GP), cardiologist, electrophysiologist, cardiac surgeon, heart failure nurse, cardiac rehabilitation team, dietician, psychologist, expert in sexual dysfunction)
Education of the patient and relatives (see Table 7.4)
Monitor progress and manage accordingly.
Reduce mortality
Reduce morbidity (to improve quality of life by relieving symptoms, increasing exercise capacity, reducing the need for hospitalization and providing end-of-life care)
Prevention (can be divided into two separate entities: (1) prevention of cardiovascular risk factors that may lead or contribute to the development of heart failure, i.e. hypertension, diabetes, obesity, and (2) prevention of progression of myocardial damage, remodelling, and reoccurrence of symptoms once HF is established).
Table 7.4 Essential topics in patient education with associated skills and appropriate self-care behaviours | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
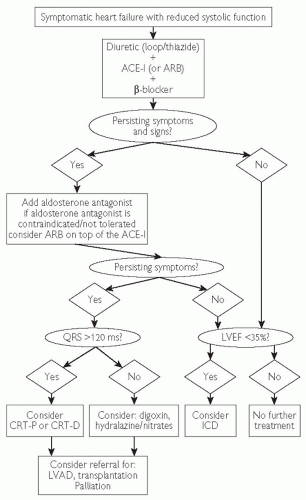 Fig. 7.4 Treatment algorithm for patients with symptomatic heart failure and reduced systolic function. Adapted from ESC (2008). Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 29: 2388-2442 and NICE guidelines  www.nice.org. ARB = angiotensin receptor blocker; LVAD = left ventricular assist device. www.nice.org. ARB = angiotensin receptor blocker; LVAD = left ventricular assist device. |
Diuretics provide symptomatic relief from pulmonary and systemic congestion by reducing fluid overload.
With the exception of aldosterone antagonists (spironalactone and eplerenone), diuretics do not offer any significant prognostic benefit.
Loop diuretics cause more pronounced diuresis (and natriuresis), and are the option of choice in patients with moderate to severe heart failure.
A thiazide may be used in combination with loop diuretics for resistant oedema. Regular monitoring is required to avoid dehydration, hyponatraemia, hypokalaemia, and hypomagnesimia.
Diuretics cause activation of the renin-angiotensin-aldosterone system and should be used in combination with an ACE-I/ARB when possible.
Start with a low dose (especially in diuretic-naive patients and the elderly) and increase the dose until clinical improvement occurs.
Once fluid overload resolves, readjust the diuretic dose to avoid dehydration. Aim to maintain ‘dry weight’ with the lowest dose possible.
Self-adjustment of the diuretic dose based on daily weight measurements and other clinical signs of fluid retention should be part of the patient education.
It is essential to monitor potassium, sodium, and creatinine levels during diuretic therapy.
Table 7.5 Considerations in the treatment of heart failure with loop diuretics | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Table 7.6 Diuretic doses used in patients with heart failure | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree