Chapter 35 Head and Neck
Normal Histology
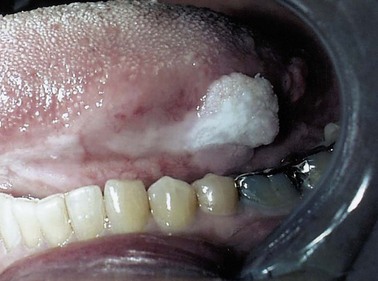
Numerous noncancerous changes in squamous epithelium can be seen in the upper aerodigestive tract. Leukoplakia, which describes any white mucosal lesion, and erythroplasia, which describes any red mucosal lesion, are both clinical descriptions and should not be used as diagnostic terms (Fig. 35-1). Erythroplakia is more often indicative of an underlying malignant lesion. Hyperplasia refers to thickening of the epithelium secondary to an increase in the total number of cells. Parakeratosis is an abnormal presence of nuclei in the keratin layers, whereas dyskeratosis refers to any abnormal keratinization of epithelial cells and is found in dysplastic lesions. Koilocytosis is a descriptive term for the vacuolization of squamous cells and is suggestive of viral infection, especially human papillomavirus (HPV).
Epidemiology
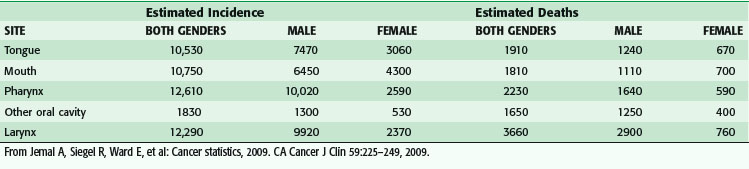
The American Joint Committee on Cancer (AJCC) staging system divides sites of malignancy originating in the head and neck into six major groups: lip and oral cavity, pharynx, larynx, nasal cavity and paranasal sinuses, major salivary glands, and thyroid.1 Of the sites arising from the aerodigestive tract, laryngeal cancer remains the most common cause of death (Table 35-1), whereas pharyngeal cancer has emerged as exhibiting the highest incidence over the past several years. Although there clearly remains a male preponderance in aerodigestive tract malignancies, the male-to-female ratio has been steadily decreasing because of the direct association between tobacco as a causative agent and the increased incidence of female smokers. Tobacco abuse increases the odds ratio for the development of laryngeal cancer by 15 : 1, whereas alcohol abuse carries an odds ratio of 2 : 1. Combined abuse of alcohol and tobacco is not additive in terms of the odds ratio but multiplicative. More recent studies have suggested that the epidemiology of head and neck cancer is shifting to mirror a change in the cause.2 In the United States, during the period from 1973 to 2003, the incidence rate for cancer sites causally related to HPV infection significantly increased (tongue base and tonsil subsites of the oropharynx), whereas significant declines in incidence were observed for oral cancers not causally related to HPV. In addition, HPV-associated cancers tended to be younger in age by 3 to 5 years and were less likely to be associated with alcohol or tobacco use. Worldwide, the highest incidence rates in males exceeded 30/100,000 in areas of France, Hong Kong, India, Spain, Italy, and Brazil, as well as in U.S. blacks, with dramatic increases in oral cancer being seen in Central and Eastern Europe, most notably Hungary, Poland, Slovakia, and Romania.3 The highest female rates are higher than 10/100,000 and are found in India, where chewing of betel quid and tobacco is common. Although aggregate rates are slowly declining in certain areas, such as India, Hong Kong, and Brazil, as well as in U.S. whites, rates are increasing in most other regions of the world. In addition to alcohol and tobacco consumption as causative factors, other risk factors include HPV and Epstein-Barr virus infection, Plummer-Vinson syndrome, metabolic polymorphisms, malnutrition, and occupational exposure to mutagenic agents. According to the National Cancer Database, squamous cell carcinoma (SCC) is the most common head and neck tumor of the major head and neck sites (88.9%), adenocarcinoma is the most common of the major salivary glands (56.4%), SCC is the most common of the sinonasal tract (43.6%), and lymphoma is the most common of the sites classified as other (82.5%).4
Carcinogenesis
HPV infection is now recognized as a causative agent for oropharyngeal carcinoma. Based on the molecular cause, HPV-positive and HPV-negative head and neck SCCs (HNSCCs) may be considered as two distinct cancers.5 High-risk HPV strains (subtypes 16 and 18) suppress apoptosis and activate cell growth when the HPV E6 and E7 proteins disrupt regulatory cell cycle and DNA repair pathways. Malignant transformation begins with inactivation of the p53 tumor suppressor gene by E6, whereas E7 inactivates the retinoblastoma tumor suppressor protein (Rb). E6 targets the cellular ubiquitin-protein ligase E6-AP, which then targets p53 for ubiquitination and degradation; this results in unregulated cell growth. E7 associates with Rb and p21 by blocking the interaction of Rb with E2F, which initiates uncontrolled cell proliferation.5 Viruses such as HPV can usurp cellular processes, but often the development of carcinoma is the result of a stepwise accumulation of genetic alterations.6 Tobacco, a well-known risk factor, was one of the first carcinogens to be linked with p53 mutations. One tobacco carcinogen, benzo[α]pyrene diol epoxide, induces genetic damage by forming covalently bound DNA adducts throughout the genome, including p53. Damage induced by benzo[α]pyrene diol epoxide and other carcinogens is repaired with the nucleotide excision repair system. Several studies have demonstrated that sequence variations in nucleotide excision repair genes contribute to HNSCC susceptibility.7
Many years after Slaughter proposed field cancerization, Califano and colleagues described the molecular basis for histopathologic changes in HNSCC.8 Samples of dysplastic mucosa and benign hyperplastic lesions displayed loss of heterozygosity at specific loci (9p21, 3p21, 17p13). In particular, loss of heterozygosity at 9p21 or 3p21 is one of the earliest detectable events leading to dysplasia in this tumor progression model. From dysplasia, further genetic alteration in 11q, 13q, and 14q results in carcinoma in situ. The high rate of recurrence of HNSCC is believed to result from histopathologically benign squamous cell epithelium harboring a clonal population with genetic alterations.8 Studies using microsatellite analysis and X chromosome inactivation have verified that metachronous and synchronous lesions from distinct anatomic sites in HNSCC often originate from a common clone. This evidence confirms that genetically altered mucosa is difficult to cure in the HNSCC patient because it is on the path to tumorigenesis, as predicted by this model. Indeed, HNSCC patients have a 3% to 7% annual incidence of secondary lesions in the upper aerodigestive tract, esophagus, or lung. A synchronous second primary lesion is defined as a tumor detected within 6 months of the index tumor. The occurrence of a second primary lesion more than 6 months after the initial lesion is referred to as metachronous. A second primary will develop in the aerodigestive tract of 14% of patients with HNSCC over the course of their lifetime, with more than half of these lesions occurring within the first 2 years of the index tumor.
There is also evidence to suggest that changes in the programming of cells, including stem cells, may also be involved in tumorigenesis in HNSCC because of the epithelial to mesenchymal transition.9 Abnormalities in cadherins, tight junctions, and desmosomes lead to a decrease in cell-cell adherence and loss of polarity, increasing the mobility of these cells. As epithelial cells disassemble their junctional structures, undergo extracellular matrix remodeling, and begin expressing proteins of mesenchymal origin, they become migratory. When the process of epithelial to mesenchymal transition becomes pathologic, regulatory checkpoints are deficient. Thus, in the carcinogenic process, epithelial to mesenchymal transition may cause changes that contribute to tumor invasion and metastasis, enabling cancer cell dissemination.9
Epidermal growth factor receptor (EGFR) signaling has been strongly implicated in tumor progression in HNSCC. The ErbB family is comprised of four structurally related receptor tyrosine kinases. EGFR mRNA and protein are preferentially expressed in HNSCC compared with surrounding normal tissues, suggesting a significant role in carcinogenesis. EGFR is overexpressed in up to 80% to 100% of HNSCC tumors, with advanced-stage and poorly differentiated carcinomas more frequently demonstrating overexpression.10 The most common mutation, EGFRvIII, occurs in up to 40% of HNSCCs. This mutant receptor is only found in cancer cells and has an in-frame deletion of exons 2 to 7, which results in a constitutively active receptor. The fact that EGFRvIII is not found in normal tissues makes this an attractive target for therapy. The two classes of therapies are monoclonal antibodies to EGFR receptor subunits and small-molecule EGFR tyrosine kinase inhibitors (TKIs). When ligands bind to one of the ErbB receptors, a dimer forms and the receptor’s intracellular tyrosine residues then undergoes ATP-dependent autophosphorylation. Once phosphorylated, the receptor has the potential to trigger many different intracellular downstream pathways. The Janus kinase–signal transducers and activators of transcription (JAK–STAT), along with the phospholipase-Cγ–protein kinase C (PLCγ–PKC) pathways are activated in association with EGFR phosphorylation.
An emerging potential target for molecular-based cancer therapy is the insulin-like growth factor-1 receptor (IGF-1R) and its ligands, insulin growth factor-1 (IGF-1) and insulin growth factor-2 (IGF-2).11 With activation of the receptor, downstream signaling events include phosphorylation of insulin receptor substrate-1 (IRS-1), activation of mitogen-activated protein kinases (MAPKs), and stimulation of the phosphatidylinositol-3 kinase (PI3K) pathway. This activation of both the Ras-MAPK-ERK and PI3K-Akt pathways is similar to the downstream signaling seen with EGFR autophosphorylation.
Staging
Staging of head and neck cancer follows the TNM classification established by the AJCC.1 The T classification refers to the extent of the primary tumor and is specific to each of the six sites of origin, with subclassifications within each site. The N classification refers to the pattern of lymphatic spread within the neck nodes and is the same for most head and neck sites, except thyroid, nasopharynx, mucosal melanoma, and skin (Table 35-2). In the new seventh edition of the AJCC Cancer Staging Manual,1 a descriptor has been added as ECS+ or ECS−, depending on the presence or absence of nodal extracapsular spread (ECS). Clinical staging of the neck is based primarily on palpation, although radiographic studies, including computed tomography (CT) and magnetic resonance imaging (MRI), have been shown to be accurate in detecting positive nodes. If the CT criteria of nodes with central necrosis or size larger than 1.0 cm are used to determine positivity, only 7% of pathologically positive lymph nodes would be missed, and these smaller nodes are most often found in necks with more extensive disease. Metastatic disease is reported simply as Mx (cannot be assessed), M0 (no distant metastases are present), or M1 (metastases present). The most common sites of distant spread are the lungs and bones, whereas hepatic and brain metastases occur less frequently. The risk for distant metastases is more dependent on nodal staging than on primary tumor size.
Table 35-2 Metastatic Staging of Regional Lymph Nodes (N)
| Stage | Description |
|---|---|
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1* | Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension |
| N2* | Metastasis in a single ipsilateral lymph node, >3 cm but not >6 cm in greatest dimension, or in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension, or in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension |
| N2a* | Metastasis in single ipsilateral lymph node >3 cm but not >6 cm in greatest dimension |
| N2b* | Metastasis in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension |
| N2c* | Metastasis in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension |
| N3* | Metastasis in a lymph node >6 cm in greatest dimension |
* A designation of U or L may be used for any N stage to indicate metastasis above the lower border of the cricoid (U) or below the lower border of the cricoid (L). Similarly, clinical or radiologic extracapsular spread (ECS) should be recorded as E− or E+, and histopathologic ECS should be designated as En (none), Em (microscopic), or Eg (gross).
From Edge SB, Byrd DR, Compton CC, et al [eds]: AJCC cancer staging manual, ed 7, New York, 2010, Springer-Verlag.
After complete resection of the primary and nodal disease, pathologic staging may be reported. This is designated by a preceding “p,” as in pTNM. It must be remembered when measuring a pathologic mucosal specimen that tumor size may decrease up to 30% after resection. Although clinical T staging is of primary concern, pathologic N staging allows detection of occult microscopic disease and is useful in determining prognosis. Site-specific staging systems are discussed according to the primary site. The major change in the 2010 edition of the AJCC staging system for HNSCC sites, in addition to the ECS + or − descriptor, is the addition of a separate classification for mucosal melanoma of the head and neck, a very rare tumor.1
Clinical Overview
Evaluation
Positron Emission Tomography
18F-fluorodeoxyglucose is a glucose analogue that is preferentially absorbed by neoplastic cells and can be detected by positron emission tomography (PET). The role of PET has been investigated in the initial evaluation of patients with HNSCC.12 PET is more sensitive than CT in identifying the primary lesion, but cannot detect unknown primary tumors with more than 50% sensitivity. More than one third of patients have a change in their TNM score based on PET findings, and 14% of patients are assigned a different stage when it is added to the diagnostic workup. PET evaluates neck metastases with sensitivity equal to that of CT but with fewer false-positive results. PET can detect a higher percentage of lung metastases than chest radiography, bronchoscopy, or CT, but the specificity ranges from 50% to 80%, and how to treat a patient with a positive PET and an otherwise negative lung workup is still in question. In approximately 10% of patients, a synchronous second primary cancer is detected in various sites, including the stomach, pancreas, colon, and thyroid. Patients with tumors that demonstrate high uptake on PET have a worse prognosis than patients with less avid tumors and also have less response to radiation therapy. The exact role of PET in the initial evaluation of HNSCC is still under investigation and its use is becoming more routine, but is not within the current standard of care.
Lymphatic Spread
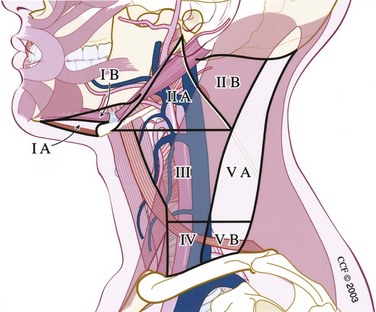
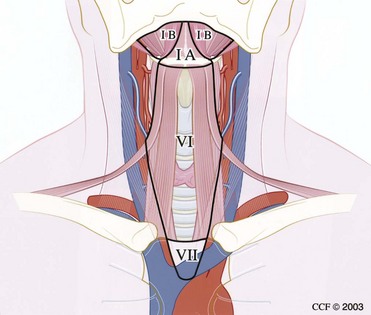
The cervical lymphatic nodal basins contain between 50 and 70 lymph nodes per side and are divided into seven levels (Figs. 35-2 and 35-3).
Therapeutic Options
Therapeutic options for patients with HNSCC include surgery, radiation therapy, chemotherapy, and combination regimens. In general, early-stage disease (stage I or II) is treated by surgery or radiation therapy. Late-stage disease (stage III or IV) is best treated by a combination of surgery and radiation therapy or chemotherapy and radiation therapy, or all three modalities, depending on the site of the primary. Because surgery was the first therapeutic option available to physicians, it has the longest track record of the three and established the head and neck surgeon as the leader of the treatment team for HNSCC. Photon irradiation is superior to surgery for eradicating microscopic disease and is an excellent alternative to surgery for early lesions. Tonsil, tongue base, and nasopharyngeal primary tumors are especially responsive to photon irradiation. Neutron and proton irradiation are used much less often in the head and neck, although experience has grown with their role in salivary gland malignancies and skull base cancers, respectively. Electrons are not commonly used in the head and neck for noncutaneous tumors. With the advent of intensity-modulated radiation therapy, which can reduce the photon dosage to surrounding normal tissue through computer three-dimensional planning, the dogma that patients may not receive more than 7200 cGy to tissue of the head and neck has been called into question. Hyperfractionation is the practice of administering radiation more than once daily, and results of the European Organization for Research and Treatment of Cancer have determined that hyperfractionation for HNSCC produces greater locoregional control than conventional once-daily regimens.13 Radiation therapy is not as effective in treating large-volume, low-grade neoplasms or tumors in close proximity to the mandible because of the risk for osteoradionecrosis. The loss of salivary function with irradiation of the oral and oropharyngeal cavity can be disabling to patients, and its impact should not be minimized in the decision making process.
A landmark chemotherapy trial for HNSCC was the Veterans Affairs larynx trial, published in 1991.14 Although chemotherapy alone is not curative in HNSCC, its role as a radiation sensitizer was established in this study. Two thirds of patients treated with radiation therapy and chemotherapy were able to keep their larynx, and survival was equal to that of patients treated with laryngectomy and radiation therapy. Recurrences after radiation therapy have been shown to be multifocal in the bed of the original tumor and the salvage surgeon should be familiar with the original tumor location and volume. Chemotherapy is commonly used in the treatment of incurable HNSCC, such as unresectable and metastatic disease, and can provide excellent symptom control in these patients.
Data from two large-scale, independent trials have examined the benefit of adding chemotherapy to postoperative irradiation for HNSCC.15,16 Both the European Organization for Research and Treatment of Cancer Trial and the Radiation Therapy Oncology Group 9501/Intergroup treated advanced-stage, high-risk patients with cisplatin concurrently with postoperative radiation therapy and compared the outcomes with those of patients undergoing postoperative irradiation alone. In the Radiation Therapy Oncology Group, the 2-year locoregional control rate was 82% for the group receiving chemoradiation therapy versus 72% for the radiation therapy–alone group. Disease-free survival was significantly longer in the chemoradiation therapy patients, although overall survival was not significantly different between the groups. Not unexpectedly, significantly more toxicity and treatment morbidity were seen in the combined-treatment group, and further prognostic indicators about which patients are at high risk for failure are needed to predict which groups warrant this more intensive adjuvant therapy.
The neck should be treated when there are clinically positive nodes or the risk for occult disease is more than 20%, based on the location and stage of the primary lesion. The decision to perform neck dissection or irradiate the neck is related to treatment of the primary lesion. If the index tumor is being treated with radiation and the neck is N0 (no clinically detectable disease) or N1, the nodes are usually treated with irradiation. For surgically treated primary lesions, N0 or N1 neck disease may also be treated surgically. Negative prognostic factors such as extracapsular spread of tumor, perineural invasion, vascular invasion, fixation to surrounding structures, and multiple positive nodes are indicators for postoperative adjuvant radiation therapy. For N2 or N3 neck disease, neck dissection with planned postoperative radiation therapy is performed. When chemoradiation therapy protocols are used in treating the primary lesion and there is a complete response in the primary tumor and an N2 or N3 neck, planned neck dissection 8 weeks after chemoradiation therapy will contain cancer in up to one third of specimens.17 If the neck mass persists, the percentage of residual disease increases to two thirds. When patients have advanced neck disease that involves the carotid artery or deep neck musculature, radiation or chemoradiation therapy is given preoperatively in the hope that the tumor will reduce in size and become resectable. CT scans notoriously carry a high false-positive rate for determining carotid encasement. When carotid resection is necessary, the associated morbidity is high (major neurologic injury in 17%), with a 22% 2-year survival rate, and the decision to resect should be weighed carefully.
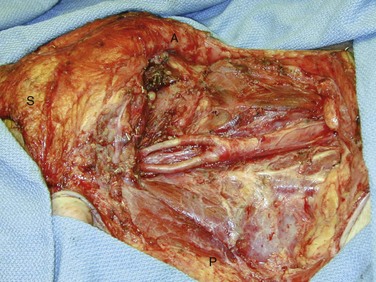
Radical neck dissection (RND) was attributed to Crile in 1906 and was considered the gold standard for the removal of nodal metastases (Fig. 35-4). Through a subsequent close reading of Crile’s surgical notes, it was found that he had begun to modify his surgical technique to remove only selected regions of the neck, depending on the site of the primary tumor. Today, this has become common surgical practice for HNSCC. All modifications of neck dissection are described in relation to the standard RND, which removes nodal levels I through V and the sternocleidomastoid muscle, internal jugular vein, cranial nerve XI, cervical plexus, and submandibular gland. Preservation of the sternocleidomastoid muscle, internal jugular vein, or cranial nerve XI in any combination is referred to as a modified RND (MRND), and the structures preserved are specified for nomenclature. A modified neck dissection may also be referred to as a Bocca neck dissection, named after the surgeon who demonstrated that not only is MRND equally as effective in controlling neck disease as RND when structures are preserved that are not directly involved in tumor, but the functional outcomes of patients after MRND are also superior to those after RND.18 Although resection of the sternocleidomastoid muscle or one internal jugular vein is relatively nonmorbid, loss of cranial nerve XI leaves a denervated trapezius muscle, which can cause a painful chronic frozen shoulder.
RND or MRND can be performed for removal of detectable nodal disease. Preservation of any of levels I through V during neck dissection is referred to as selective neck dissection and is based on knowledge of the patterns of spread to neck regions. Selective neck dissection is performed on a clinically negative (N0) neck, with preservation of nodal groups carrying less than a 20% chance of being involved with metastatic disease. Regional control has been shown to be as effective after selective neck dissection as after MRND in patients with a clinically negative neck. Recent studies evaluating treatment of an N0 neck have investigated the use of sentinel lymph node biopsy, which attempts to predict the disease status of the neck based on the first echelon of nodes that drain the tumor.19 Although sentinel lymph node biopsy has been used extensively with melanoma, its use in HNSCC has come about more gradually. Early results using isosulfan blue dye alone have suggested that this technique cannot consistently identify the sentinel node in HNSCC. More recent results using a gamma probe have been more encouraging, although the isolated node should be serial step–sectioned at a thickness of 150 nm and be examined through permanent processing. The current recommendations are that the technique should be restricted to early-stage (T1 or T2) oral and oropharyngeal cancers, with clinically N0 necks; this continues to be an investigational tool pending validation by large randomized clinical trials.
Anatomic Sites
Lip
The most common clinical manifestation of lip cancer is an ulcerative lesion on the vermilion or skin surface. Palpation is necessary to determine the submucosal extent of the lesion and possible fixation to underlying bone. Sensation of the chin should be tested to determine involvement of the mental nerve. Poor prognostic indicators include nerve involvement, fixation to the maxilla or mandible, cancer arising on the upper lip or commissure, positive nodal disease, and age younger than 40 years at diagnosis. The most frequently involved nodal basins are the submental and submandibular levels. A depth of tumor invasion of 4 mm has been shown to be a cutoff above which the incidence of cervical nodal disease is significantly increased.20
Similar to the rest of the oral cavity, lip cancer staging is based on size at initial evaluation. Early-stage disease may be treated by surgery or radiation therapy with equal success. Local surgery (wide local excision) with negative margin control of at least 3 mm is the preferred treatment, with supraomohyoid neck dissection performed for tumors with clinically negative necks but deeper primary invasion or size larger than 3 cm. Neck dissection with postoperative radiation therapy for patients with clinically evident neck disease has an acceptable 91% regional control rate in the neck.21 The overall 5-year cure rate of 90% drops to 50% in the presence of neck metastases. Postoperative irradiation is also indicated for advanced-stage primary disease, tumors with perineural involvement, or close or positive margins at the time of resection.
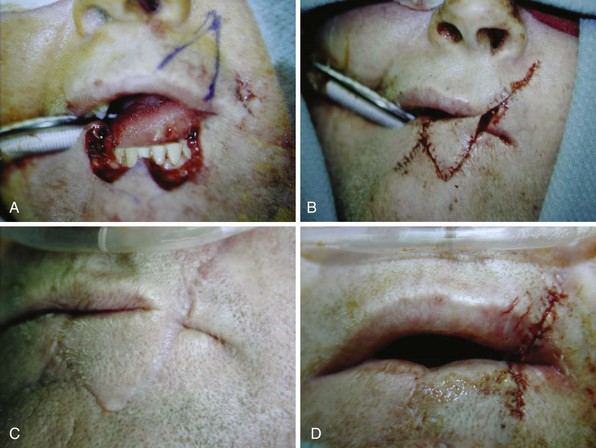
Defects encompassing between half and two thirds of the lip require augmentation. The Estlander and Abbé flaps are lip switch flaps based on the sublabial or superior labial artery. The Estlander flap is used when the defect involves the commissure, whereas the Abbé flap is used for more midline defects and requires second-stage division of the pedicle (Fig. 35-5). The Karapandzic flap consists of circumoral incisions with circular rotation of the skin flaps while maintaining innervation of the orbicularis oris musculature. This one-stage procedure is used for defects involving more than two thirds of the lip. Microstomia is a potential complication from these types of flap reconstructions, and denture use may not be possible. For defects larger than two thirds, the Webster, Gillies, or Bernard types of repairs may also be used.
Oral Cavity
Floor of the Mouth
Bimanual palpation can often determine fixation of tumors of the floor of the mouth to the mandible. CT demonstrates the depth of mandibular bony invasion, and widening of the cranial neural foramen, such as the foramen ovale, suggests neurotropic intracranial spread in advanced tumors. Determining mandibular invasion is of utmost importance for preoperative planning (Fig. 35-6). Invasion into the tongue musculature necessitates partial glossectomy concurrently with removal of the lesion on the floor of the mouth.
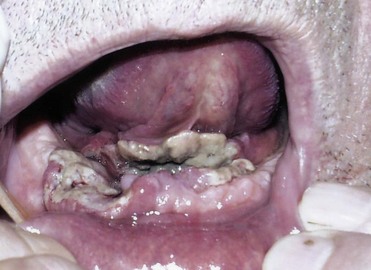
FIGURE 35-6 62-year-old man with squamous cell carcinoma of the anterior floor of mouth invading the mandible.
Buccal Mucosa
The buccal mucosa extends from the inner surface of the opposing surfaces of the lips to the alveolar ridges and pterygomandibular raphe. Buccal cancer is uncommon and represents 5% of all oral cavity carcinomas. Smoking, alcohol abuse, lichen planus, dental trauma, snuff dipping, and tobacco chewing are causative factors associated with buccal cancer. Approximately 65% of patients with buccal cancer are initially found to have extension beyond the cheek mucosa. Lymphatic drainage is to the submandibular lymph nodes; however, tumors in the posterior aspect of the cheek may spread to level II initially. Stage I cancers have historically been treated by surgery and did not involve elective neck dissection because of the low rate of occult metastases. More recent studies, however, have suggested high rates of local recurrence for lesions treated by surgery alone, and adjuvant radiation therapy has been suggested, even for early-stage lesions.22 Deep invasion may require through and through excision of cheek skin, thus necessitating internal and external linings, usually with a fasciocutaneous free flap.
Oropharynx
Treatment of oropharyngeal SCC has focused increasingly on conservation therapy with chemotherapy and radiation therapy. Many tumors of the oropharynx are poorly differentiated and respond well to radiation. Chemotherapy has been used as a radiation sensitizer in a number of studies and the local control rate achieved has been 90%, even in stage IV disease, although overall survival has not improved over more traditional surgery and radiation therapy.23 A recent study of the cause of tonsil and tongue base cancers has suggested that when the disease is associated with HPV infection, the prognosis is significantly improved over non-HPV tumors. In a phase II trial of investigational therapy in patients with oropharyngeal and laryngeal cancers (ECOG 2399), patients with HPV positive tumors had a 73% reduction in risk of progression and a 64% reduction in risk of death when compared with HPV-negative patients.24 This landmark study was the first to demonstrate that tumor HPV status is a strong and favorable prognostic marker in uniform patient populations with similar treatment protocols. Many physicians are advising that tumor HPV status should be incorporated as a stratification factor in patients with oropharyngeal cancer, although basing treatment protocols on HPV status has yet to be definitively investigated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree