Percutaneous mitral valve repair using the MitraClip device has become a therapeutic alternative for high surgical risk patients with symptomatic mitral regurgitation. The procedure involves transseptal puncture and results in a new atrial septal defect (ASD) after withdrawal of the 22Fr guiding catheter. The functional effect of the new ASD is not defined. In 28 patients with symptomatic mitral regurgitation undergoing percutaneous mitral valve repair using the MitraClip device, 3-dimensional transesophageal echocardiography was used to measure by direct en face imaging the area of the new ASD. Analysis of the velocity-time integral (VTI) across the ASD after withdrawal of the guiding catheter allowed calculation of the shunt volume. Diastolic VTI of the mitral flow was determined before and after withdrawal of the guiding catheter to determine left ventricular inflow changes. Invasive left atrial pressure measurements were obtained during withdrawal of the guiding catheter. Regurgitant volume was reduced from 86 ± 21 ml/beat before intervention to 43 ± 22 ml/beat after intervention. The new ASD had an area of 0.19 cm 2 , 44% of the area of the 22Fr guiding catheter. Considering the VTI across the septal defect of 72 ± 26 cm/s, the left-to-right atrial shunt volume was calculated to be 14 ± 6 ml/beat. The diastolic forward flow across the mitral valve was reduced by 13 ± 6 ml/beat immediately after withdrawal of the MitraClip guiding catheter. Mean left atrial pressure was reduced from 17 ± 8 mm Hg with the guiding catheter still in the left atrium to 15 ± 8 mm Hg after withdrawal of the guiding catheter. In conclusion, the creation of a new ASD as consequence of the large-diameter MitraClip guiding catheter results in volume and pressure relief of the left atrium. This contributes to the immediate hemodynamic changes implemented by the MitraClip procedure.
Percutaneous mitral valve repair using the MitraClip device (Abbott Vascular Structural Heart, Menlo Park, California) has become a therapeutic alternative for high surgical risk patients with symptomatic mitral regurgitation. A significant reduction of the left ventricular (LV) filling pressure and other hemodynamic consequences have been reported after the procedure. The procedure involves transseptal puncture with insertion of a 22Fr guiding catheter and results in a new atrial septal defect (ASD) after withdrawal of this guiding catheter. The functional and hemodynamic effects of the new ASD are not defined. Three-dimensional (3D) transesophageal echocardiography (TEE) allows accurate analysis of ASD size. This study aimed at evaluating the size of the new ASD, calculating the ASD shunt volume, defining the change in left atrial pressure due to the new ASD after the MitraClip procedure.
Methods
Twenty-eight consecutive high-risk or inoperable patients with symptomatic mitral regurgitation were included in this study. Seventeen patients were considered to have functional mitral regurgitation and 11 patients were considered to have a predominantly degenerative cause.
Percutaneous mitral valve repair using the MitraClip device was performed as previously described in detail. After transseptal puncture, a 22Fr guiding catheter was advanced from the right atrium to the left atrium. After deployment of one or several MitraClip devices, the guiding catheter was drawn back from the left atrium to the right atrium. A new ASD remained at the site where the MitraClip guiding catheter had been inserted through the interatrial septum.
Three-dimensional TEE studies were performed with a commercially available echocardiographic system (Vivid E9, General Electric Vingmed, Horten, Norway, or iE33, Philips Medical Systems, Andover, Massachusetts) and the corresponding 3D TEE probe throughout the procedure to guide every interventional step including definition of the mitral valve area as well as mitral regurgitation vena contracta area and regurgitation volume before and after the MitraClip procedure. After retrieval of the MitraClip guiding catheter, 3D TEE was used to measure by direct en face imaging the area of the new ASD, to determine the resulting left-to-right atrial shunt volume, and to define the concomitant reduction of forward flow through the mitral valve due to the new ASD.
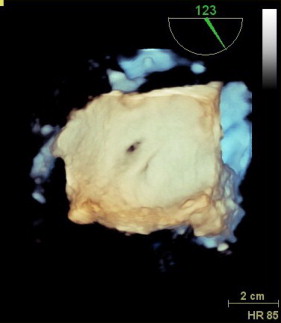
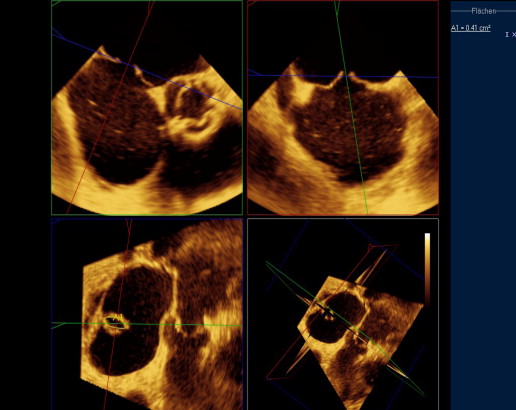
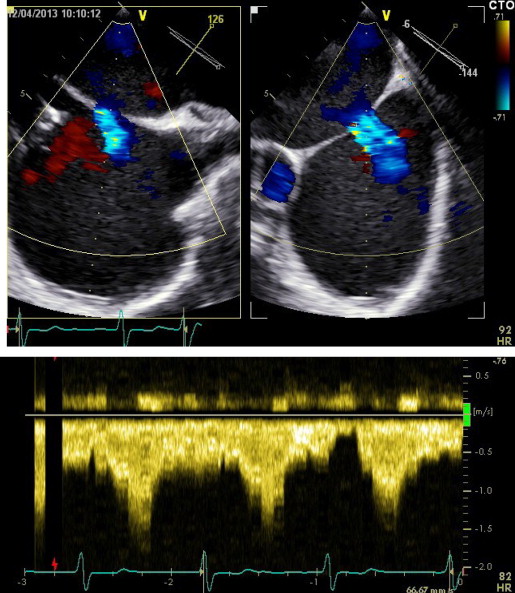
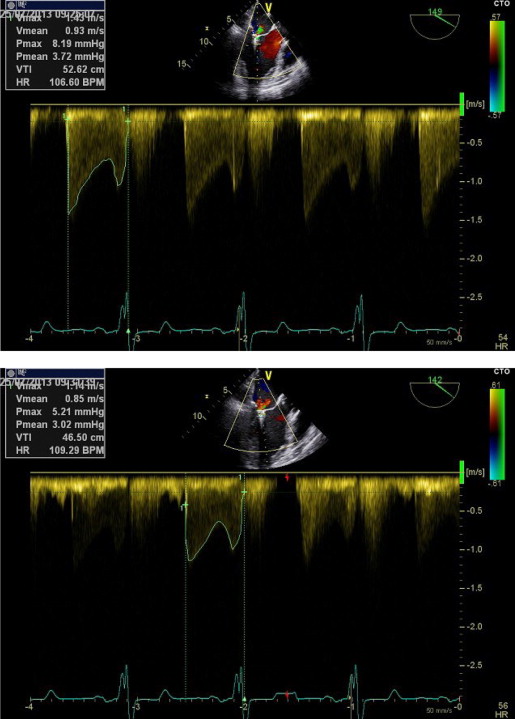
Three-dimensional TEE data acquisition was performed over 4 heart cycles with typical volume rates of 24 to 28/s ( Figure 1 ). Analysis of data sets was performed offline on the echocardiographic systems. The cropping tool allowed adjusting 2 known views to help with orientation. Orthogonal to these 2 helping views, a third plane was placed allowing to directly visualize the ASD orifice area in this cross-sectional view. Planimetry was performed in this view to define the maximal ASD orifice area during a cardiac cycle ( Figure 2 ). Considering the determined ASD area and the analysis of the velocity-time integral (VTI) using continuous wave Doppler across the ASD after withdrawal of the guiding catheter ( Figure 3 ) allowed calculation of the shunt volume. Analysis of the diastolic mitral flow was performed before and after withdrawal of the MitraClip guiding catheter to determine the change in LV inflow induced by opening the ASD ( Figure 4 ). It was calculated as the product of the sum of each mitral valve area (lateral and medial to the MitraClip) measured by 3D TEE direct planimetry and VTI of diastolic mitral flow using continuous wave Doppler as described before. Mitral valve regurgitant volume by color Doppler 3D TEE was determined as the product of vena contracta areas defined by direct planimetry and VTI of the regurgitant jet using continuous wave Doppler. In case of atrial fibrillation, all echocardiographic measurements were performed on 5 consecutive cardiac cycles and subsequently averaged. There was an 8 ± 3% intraobserver variation and a 10 ± 3% interobserver variation on ASD area.
To obtain invasive left atrial pressure measurements during withdrawal of the MitraClip guiding catheter and thereby measure the change in left atrial pressure after opening the newly created ASD, a 120-cm long 5Fr multipurpose catheter was advanced through the MitraClip guiding catheter until the tip entered the left atrium. The multipurpose catheter was connected to an external pressure transducer for continuous pressure monitoring ( Figure 5 ). Subsequently, the tip of the multipurpose catheter was kept in the left atrium while the tip of the MitraClip guiding catheter was withdrawn to the right atrium during respiratory hold. In case of atrial fibrillation, pressure curves of 5 consecutive cardiac cycles with the catheter in the left and right atria were evaluated and averaged.
Statistical analysis was performed using SPSS version 15.0 (SPSS, Inc., Chicago, Illinois). Categorical data are presented as frequencies and were compared using Fischer’s exact test. Continuous data are presented as mean ± SD and were compared using the Student t test or analysis of variance, as appropriate. A p value of <0.05 was considered statistically significant.
Results
The relevant echocardiographic and invasive measurements of all patients are given in Table 1 . The mitral valve regurgitant volume, calculated as the product of vena contracta area defined by direct planimetry and systolic regurgitant VTI using continuous wave Doppler, was reduced from 86 ± 31 ml/beat before intervention to 43 ± 22 ml/beat after intervention. Three-dimensional TEE defined the new ASD to have an effective area of 0.19 ± 0.05 cm 2 . Thus, compared with the cross-sectional area of 0.43 cm 2 of the 22Fr MitraClip guiding catheter, the new ASD had a relative cross-sectional area of 44%. Using continuous wave Doppler echocardiography, a VTI across the ASD of 72 ± 26 cm/s was measured. The left-to-right atrial shunt volume calculated as the product of effective ASD area and VTI across the ASD was found to be 14 ± 6 ml/beat. Considering the heart rate after withdrawal of the guiding catheter, this amounted to a shunt volume of 1,218 ± 567 ml/min. Simultaneous to the withdrawal of the MitraClip guiding catheter, the diastolic forward flow across the mitral valve was reduced by 13 ± 6 ml/beat. Invasive pressure measurements showed that the mean left atrial pressure was reduced from 17 ± 8 mm Hg with the guiding catheter still in the left atrium to 15 ± 8 mm Hg after withdrawal of the guiding catheter.
| Age (yrs) | MR Grade | MR Volume Pre (ml) | ASD Area (cm 2 ) | VTI ASD (cm) | Shunt ASD (ml) | LV Inflow With Guiding (ml/beat) | LV Inflow Without Guiding (ml/beat) | Δ LV Inflow (ml/beat) | Pressure LA Pre (mm Hg) | Pressure LA Post (mm Hg) |
|---|---|---|---|---|---|---|---|---|---|---|
| 61 | III | 69 | 0.12 | 75 | 9 | 68 | 58 | 10 | 10 | 7 |
| 65 | III | 112 | 0.14 | 86 | 12 | 78 | 65 | 13 | 16 | 11 |
| 67 | III | 53 | 0.15 | 99 | 15 | 119 | 103 | 16 | 10 | 8 |
| 69 | IV | 65 | 0.22 | 82 | 18 | 167 | 140 | 27 | 10 | 9 |
| 71 | III | 86 | 0.13 | 74 | 10 | 67 | 60 | 7 | 14 | 12 |
| 71 | III | 54 | 0.25 | 52 | 13 | 111 | 100 | 11 | 26 | 22 |
| 72 | II | 78 | 0.15 | 56 | 8 | 57 | 48 | 10 | 14 | 11 |
| 74 | III | 60 | 0.18 | 55 | 10 | 145 | 131 | 14 | 26 | 23 |
| 75 | IV | 117 | 0.20 | 84 | 17 | 87 | 69 | 18 | 19 | 18 |
| 75 | III | 67 | 0.22 | 79 | 17 | 167 | 147 | 20 | 10 | 8 |
| 76 | IV | 105 | 0.16 | 60 | 10 | 137 | 125 | 12 | 12 | 8 |
| 76 | III | 49 | 0.15 | 53 | 8 | 99 | 89 | 10 | 18 | 16 |
| 77 | IV | 104 | 0.21 | 68 | 14 | 79 | 66 | 13 | 28 | 26 |
| 79 | III | 74 | 0.28 | 55 | 15 | 121 | 104 | 17 | 10 | 8 |
| 80 | III | 80 | 0.25 | 97 | 24 | 119 | 99 | 20 | 24 | 21 |
| 80 | III | 95 | 0.26 | 82 | 21 | 133 | 114 | 19 | 14 | 14 |
| 80 | III | 63 | 0.16 | 171 | 27 | 119 | 94 | 25 | 17 | 13 |
| 82 | III | 98 | 0.20 | 72 | 14 | 88 | 76 | 12 | 12 | 11 |
| 82 | IV | 74 | 0.21 | 59 | 12 | 134 | 131 | 3 | 21 | 18 |
| 83 | III | 83 | 0.16 | 71 | 11 | 114 | 101 | 13 | 36 | 37 |
| 83 | IV | 113 | 0.18 | 92 | 17 | 97 | 83 | 14 | 16 | 12 |
| 85 | III | 68 | 0.17 | 56 | 9 | 105 | 96 | 9 | 28 | 26 |
| 85 | IV | 99 | 0.26 | 94 | 24 | 130 | 105 | 25 | 15 | 15 |
| 85 | III | 89 | 0.27 | 59 | 16 | 141 | 123 | 17 | 18 | 14 |
| 87 | III | 54 | 0.20 | 65 | 13 | 139 | 126 | 13 | 17 | 14 |
| 87 | III | 96 | 0.17 | 44 | 7 | 102 | 95 | 7 | 11 | 10 |
| 87 | III | 75 | 0.15 | 65 | 10 | 85 | 72 | 13 | 22 | 19 |
| 89 | IV | 105 | 0.16 | 22 | 3 | 92 | 87 | 5 | 11 | 8 |
| Mean ± SD | 86 ± 21 | 0.19 ± 0.05 | 72 ± 26 | 14 ± 6 | 111 ± 29 | 97 ± 27 | 13 ± 6 | 17 ± 8 | 15 ± 8 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


