The risk factors for superior vena cava (SVC) obstruction after pediatric orthotopic heart transplantation (OHT) have not been identified. This study tested the hypothesis that pretransplant superior cavopulmonary anastomosis (CPA) predisposes patients to SVC obstruction. A retrospective review of the Pediatric Cardiac Care Consortium registry from 1982 through 2007 was performed. Previous CPA, other cardiac surgeries, gender, age at transplantation, and weight at transplantation were assessed for the risk of developing SVC obstruction. Death, subsequent OHT, or reoperation involving the SVC were treated as competing risks. Of the 894 pediatric OHT patients identified, 3.1% (n = 28) developed SVC obstruction during median follow-up of 1.0 year (range: 0 to 19.5 years). Among patients who developed SVC obstruction, 32% (n = 9) had pretransplant CPA. SVC surgery before OHT was associated with posttransplant development of SVC obstruction (p <0.001) after adjustment for gender, age, and weight at OHT and year of OHT. Patients with previous CPA had increased risk for SVC obstruction compared with patients with no history of previous cardiac surgery (hazard ratio 10.6, 95% confidence interval: 3.5 to 31.7) and to patients with history of non-CPA cardiac surgery (hazard ratio 4.7, 95% confidence interval: 1.8 to 12.5). In conclusion, previous CPA is a significant risk factor for the development of post–heart transplant SVC obstruction.
Orthotopic heart transplantation (OHT) is used successfully for patients with end-stage cardiac failure or irreparable congenital heart disease. Superior vena cava (SVC) obstruction has emerged as a notable complication following OHT, particularly with the bicaval method of graft implantation. It has been postulated that younger age at transplantation, donor–recipient caval mismatch, and previous surgery involving the SVC, and previous superior cavopulmonary anastomosis (CPA) may be risk factors for posttransplant SVC obstruction. However, the incidence of SVC obstruction in pediatric heart transplant patients and the effect of pretransplant exposure to previous superior CPA or other types of non-CPA SVC surgeries remain unknown. This study describes the incidence of SVC obstruction in pediatric transplant recipients from a large multicenter database and investigates its association with previous cardiac surgeries and other risk factors. We hypothesized that patients with previous superior CPA, younger age, and smaller weight at transplantation would indicate higher risk for SVC obstruction.
Methods
We conducted a retrospective cohort study using data from the Pediatric Cardiac Care Consortium (PCCC), a voluntary multi-institutional registry collecting outcome data after cardiac procedures, including surgeries and cardiac catheterizations, for pediatric or adult congenital heart diseases. The registry includes data from 50 institutions in United States and Canada. All cardiac catheterizations and operations performed during the years a center was participating in PCCC are included in the database. Each site maintains its own evaluation and management approach for heart transplantation. Details about the nature and operation of the PCCC have been described before. The study was approved by the Institutional Review Board at the University of Minnesota without requirement for patient consent. We identified pediatric patients (<18 years at transplantation) who underwent OHT in a PCCC center from 1982 to 2007. Data reviewed included age and weight at transplant, patient’s gender, diagnoses, and types and timing of interventional procedures. CPA was defined as any superior CPA, regardless of whether simultaneous or subsequent inferior CPA was performed. Patients without CPA were grouped by whether they had had previous cardiac surgery. We considered that patients with non-CPA surgery involving the SVC, such as partial anomalous pulmonary vein repair and atrial baffle procedures might also be at increased risk, but these represented only 3.8% of our cohort, and none developed SVC obstruction, so we grouped them with other non-CPA cardiac surgery patients in the analysis. Diagnosis of SVC stenosis was made by the center’s managing team and may have included a combination of clinical, imaging, and hemodynamic data, which may not be available to PCCC. All diagnoses of SVC obstruction were included when made at the time of the first cardiac procedure reported in the PCCC (either cardiac catheterization or surgery), independent of when or whether an intervention to relieve took place. Cardiac catheterizations were performed at the discretion of the treating team, either as a routine posttransplantation surveillance, or secondary due to other clinical indications. SVC obstructions identified in autopsy were not included in this study.
Among 90,124 patients operated in the PCCC, 971 pediatric cases with OHT were identified. Only the first OHT per patient was included. We excluded patients with incomplete or conflicting data (n = 22), pretransplant SVC obstruction (n = 11), first OHT outside of PCCC (n = 9), left or bilateral SVCs, as well as patients requiring additional SVC-related surgery at the time of transplant, such as partial anomalous pulmonary venous repair (n = 10) or placed on mechanical circulatory support (n = 23) and patients who met multiple exclusion criteria (n = 2). After exclusions 894 patients were available for analysis (age range: 1 day to 18 years, median: 3.4 years).
Primary outcome was time from OHT to SVC obstruction before death, subsequent OHT, or reoperation involving the SVC. Follow-up data were available each time a patient had a subsequent cardiac procedure in a PCCC center. Because of the multiple outcomes following OHT, we used a competing risk analysis with SVC obstruction, death, retransplantation, and additional nontransplant cardiac surgery treated as competing risks. These competing risks were used to analyze the relationship between SVC obstruction and CPA or other previous cardiac surgery, adjusting for gender, age at OHT, weight at OHT, and decade of OHT. Statistical significance was tested using Wald chi-square statistics at the p <0.05 level. Age, year of OHT, and weight were allowed to be nonlinear using restricted cubic splines, with 3 knots each set at the 10th, 50th, and 90th percentiles. The proportional hazard assumption was assessed by plotting scaled Schoenfeld residuals. The hazard ratios (HRs) and 95% confidence intervals (CIs) comparing the 75th percentile and the 50th percentile to the 25th percentile for each continuous variable were computed along with the HR and 95% CI for the categorical variables.
Results
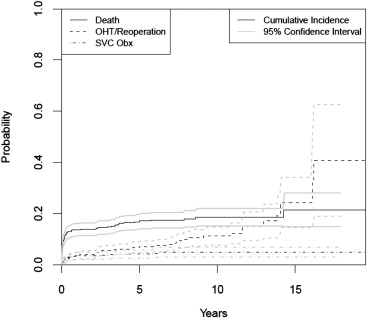
The characteristics of patients undergone OHT are described in Table 1 . The median follow-up time was 1 year (range: 0 to 19.5 years). During follow-up, 28 patients (3%) developed SVC obstruction, 122 (14%) patients died, 57 (6%) had a second OHT or a subsequent surgery involving the SVC, and 687 (77%) were observed for variable lengths of time without reaching any of these end points. The cumulative incidence of SVC obstruction in OHT patients was 2.6% (95% CI: 1.4 to 3.7) at 6 months, 3.4% (95% CI: 2.0 to 4.7) at 1 year, 3.6% (95% CI: 2.2 to 5.0) at 3 years, and 4.1% (95% CI: 2.5 to 5.7) at 5 years ( Figure 1 ). Characteristics of patients with and without SVC obstruction are available in Table 2 .
| Characteristics | n (%) ∗ |
|---|---|
| Gender | |
| Male | 494 (55) |
| SVC-related surgery before OHT | |
| Previous CPA | 84 (9%) |
| Previous SVC surgery/other | 284 (32%) |
| Age at OHT (yrs), median (min, max) | 3.4 (0, 17.9) |
| Weight at OHT (kg), median (min, max) | 12.9 (2.1, 113) |
| Decade | |
| 1982–1989 | 52 (6%) |
| 1990–1999 | 411 (46%) |
| 2000–2007 | 431 (48%) |
| Total | 894 |
| Variable | SVC Obstruction (n = 28) | Death (n = 122) | OHT/Reoperation (n = 57) | No Event (n = 687) |
|---|---|---|---|---|
| Age at OHT (yrs) | 4.9 ± 6.4, 0.85 | 4.8 ± 6.1, 1.3 | 5.4 ± 5.3, 4.2 | 6.7 ± 6.3, 4.1 |
| Weight at OHT (kg) | 16.3 ± 16.1, 9.4 | 18.1 ± 21.2, 8.3 | 20.7 ± 22.5, 12.0 | 23.9 ± 21.5, 14.7 |
| Male | 16 (57%) | 65 (53%) | 30 (53%) | 383 (56%) |
| Previous CPA | 9 (32%) | 14 (11%) | 7 (12%) | 54 (8%) |
| Previous SVC/other surgery | 9 (32%) | 36 (30%) | 20 (35%) | 219 (32%) |
| Decade | ||||
| 1982–1989 | 2 (7%) | 16 (13%) | 7 (12%) | 27 (4%) |
| 1990–1999 | 9 (32%) | 63 (52%) | 33 (58%) | 306 (45%) |
| 2000–2007 | 17 (61%) | 43 (35%) | 17 (30%) | 354 (52%) |
History of surgery involving the SVC before OHT was associated with an increased risk of SVC obstruction following OHT (p = 0.001) after adjustment for gender, age, and weight at OHT and year of OHT ( Table 3 ). Furthermore, age appeared to have a nonlinear association with risk of SVC obstruction following OHT. There was no consistent relationship between risk of SVC obstruction and age or weight. Patients who underwent CPA were at significantly higher risk of SVC obstruction compared with those patients with no pre-OHT cardiac surgical history (HR: 10.6, 95% CI: 3.5 to 31.7; Table 4 ). Similarly, CPA patients had a higher risk of SVC obstruction after transplant than patients with a history of all other types of cardiac surgery (HR: 4.7, 95% CI: 1.8 to 12.5). Patients who underwent non-CPA cardiac surgery were at higher risk of SVC obstruction compared with those patients with no cardiac surgical history (HR: 2.2, 95% CI: 0.79 to 6.4), although this was not statistically significant ( Table 4 ). From the non-CPA patients who developed SVC obstruction after OHT (n = 19), 13 had undergone bicaval technique versus 5 biatrial, and in one case, no information was available about the surgical technique. However, without knowing the overall number of patients undergoing bicaval versus biatrial technique, this information cannot infer relationship with SVC obstruction. Of the 28 patients with SVC obstruction following OHT, 54% (n = 15) of the patients required SVC intervention. Of these patients, 73% (n = 11) underwent percutaneous intervention (balloon angioplasty, n = 2; or stent placement, n = 9) and 27% (n = 4) underwent surgical patch augmentation ( Table 5 ).
| Variable | Chi-Square | df | p Value |
|---|---|---|---|
| History of surgery involving SVC before OHT | 19.10 | 2 | 0.0010 ∗ |
| Gender | 0.03 | 1 | 0.8701 |
| Age (yrs) | 8.02 | 2 | 0.0181 ∗ |
| Nonlinear component | 3.22 | 1 | 0.0730 |
| Surgical weight (kg) | 4.48 | 2 | 0.1062 |
| Nonlinear | 0.18 | 1 | 0.6689 |
| Yr of transplant | 1.74 | 2 | 0.4180 |
| Nonlinear | 1.30 | 1 | 0.2548 |
| Total Nonlinear | 9.33 | 3 | 0.0252 |
| Total | 26.59 | 7 | 0.0004 |
| Variable | HR | Lower 95% CI | Upper 95% CI |
|---|---|---|---|
| History of surgery associated with SVC before OHT | |||
| CPA vs none | 10.6 ∗ | 3.5 | 31.7 |
| CPA vs SVC surgery/other | 4.7 ∗ | 1.8 | 12.5 |
| SVC surgery/other vs none | 2.2 | 0.79 | 6.4 |
| Gender | |||
| Female vs male | 1.1 | 0.50 | 2.3 |
| Age | |||
| 50th vs 25th percentile (3.4 vs 0.4 yrs) | 0.27 | 0.03 | 2.5 |
| 75th vs 25th percentile (13.1 vs 0.4 yrs) | 0.79 | 0.01 | 43.5 |
| Surgical weight | |||
| 50th vs 25th percentile (12.9 vs 5.4 kg) | 0.97 | 0.19 | 5.11 |
| 75th vs 25th percentile (37.5 vs 5.4 kg) | 0.43 | 0.01 | 23.3 |
| Transplant yr | |||
| 50th vs 25th percentile (1999 vs 1995) | 1.49 | 0.82 | 2.71 |
| 75th vs 25th percentile (2004 vs 1995) | 1.27 | 0.57 | 2.85 |
| Patient | Diagnosis | Pre-OHT Surgeries | Pre-OHT Risk | OHT Era ∗ | Wt (kg) at OHT | OHT Technique | Age (yrs) at OHT | Age (yrs) at dx of SVC | Intervention(s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PAIVS | Shunt, Glenn + ECMO, shunt + ECMO | Glenn | 3 | 10 | Bicaval | 0.5 | 1 | Stent, balloon |
| 2 | TGA, mitral stenosis | DKS + Glenn | Glenn | 3 | 23 | Bicaval | 0.8 | 0.8 | Stent |
| 3 | Tricuspid atresia, coarctation of aorta | Norwood, Glenn | Glenn | 2 | 8 | Bicaval | 0.9 | 1.4 | Stent |
| 4 | Heterotaxy, complex SV, TAPVC | TAPVC repair, Glenn | Glenn | 2 | 15 | Unknown | 7 | 7.8 | Stent |
| 5 | Complex SV, coarctation of aorta | Glenn, Fontan, multiple reoperations | Glenn | 3 | 26 | Bicaval | 10 | 10.3 | None as of 9 mo f/u |
| 6 | Heterotaxy, complex SV, intIVC | Glenn, Fontan | Glenn | 3 | 36 | Bicaval | 10.3 | 12.8 | None as of 3 yr f/u |
| 7 | Heterotaxy, complex SV, TAPVC | Glenn, AV valve repair | Glenn | 3 | 27 | Bicaval | 15.6 | 15.7 | None at dx |
| 8 | HLHS | DKS, Glenn, Fontan, ASD creation | Glenn | 3 | 34 | Bicaval | 15.6 | 15.7 | None as of 4 mo f/u |
| 9 | Heterotaxy, complex SV, intIVC | Kawashima | Glenn | 3 | 51 | Bicaval | 16.9 | 17.1 | Stent |
| 10 | Unbalanced AVC, arch hypoplasia | Norwood | Cardsurg | 3 | 4 | Bicaval | 0.1 | 0.1 | Surgical patch |
| 11 | Complex SV, coarctation of aorta | Create ASD+VSD, AVVR | Cardsurg | 2 | 4 | Atrial | 0.3 | 0.8 | Surgical patch |
| 12 | Complex SV, arch hypoplasia | Multiple arch repairs | Cardsurg | 2 | 5 | Atrial | 0.4 | 0.4 | None as of 1 mo f/u |
| 13 | HLHS | Norwood | Cardsurg | 1 | 3 | Atrial | 0.5 | 6.4 | None as of 10 yr f/u |
| 14 | Multiple VSDs, coarctation of aorta | VSD repair | Cardsurg | 2 | 6 | Bicaval | 0.8 | 0.8 | Balloon, stent |
| 15 | Situs inversus, DORV, left SVC | VSD baffle and RVOT patch | Cardsurg | 2 | 10 | Bicaval | 1.6 | 1.6 | Surgical patch |
| 16 | Heterotaxy, partial AVC, intIVC | ASD repair, AV valve replacement | Cardsurg | 3 | 9 | Bicaval | 1.7 | 2.3 | None at dx |
| 17 | IAA/VSD, subAS | IAA/VSD repair, Ross-Konno, CABG | Cardsurg | 3 | 15 | Atrial | 5.9 | 6.3 | None as of 9 mo f/u |
| 18 | Complex SV | Fontan | Cardsurg | 3 | 45 | Bicaval | 16.1 | 16.9 | None as of 4 mo f/u |
| 19 | HLHS | Fontan | Cardsurg | 3 | 11 | Bicaval | 2.4 | 2.5 | None as of 7 yr f/u |
| 20 | PAIVS | 3 | 4 | Bicaval | 0 | 0.3 | Surgical patch, stent | ||
| 21 | HLHS | 3 | 3 | Unknown | 0.1 | 3.2 | None as of 3 yr f/u | ||
| 22 | HLHS | 1 | 3 | Atrial | 0.1 | 3.7 | Stent, balloon | ||
| 23 | Cardiomyopathy | 3 | 5 | Bicaval | 0.2 | 0.4 | Balloon | ||
| 24 | HLHS | 2 | 4 | Bicaval | 0.2 | 6.3 | None as of 3 yr f/u | ||
| 25 | HLHS | 2 | 4 | Bicaval | 0.2 | 0.6 | Stent, stent | ||
| 26 | Cardiomyopathy | 3 | 6 | Bicaval | 0.3 | 0.9 | None as of 4 yr f/u | ||
| 27 | Cardiomyopathy | 2 | 44 | Bicaval | 13.2 | 13.5 | Stent | ||
| 28 | Pulmonary hypertension | 3 | 52 | Bicaval | 16.3 | 16.5 | Stent |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


