Lung transplantation, which involves an anastomosis of the graft to the native left atrium, may increase the risk of left-side atrial flutter (AFL). Our aim was to evaluate the incidence, predisposing conditions, and course of AFL after lung transplantation in adults. Two hundred sixty-nine consecutive patients who underwent lung transplantation were studied retrospectively. All patients received a preoperative echocardiogram and postoperative electrocardiographic monitoring. All 12-lead electrocardiograms were reviewed. Typical or atypical AFL was diagnosed by 2 independent reviewers based on accepted criteria. Predictors of AFL were investigated separately using univariate and multivariate logistic regression analyses. AFL occurred in 35 of 269 patients (13%) over a mean of 12 days after transplantation. All patients who developed AFL had no previous atrial arrhythmia. Of these 35 patients, 24 (68.6%) had atypical AFL by electrocardiographic criteria. In multivariate logistic regression analysis, patients with idiopathic pulmonary fibrosis (IPF) were 2.9 times more likely to have AFL than those patients with lung transplant without IPF (p = 0.009). Other independent risk factors for AFL were advanced age and preoperative left atrial enlargement. Only 3 of 35 patients (8.6%) with AFL had persistent atrial arrhythmia and needed electrophysiologic study and ablation. In conclusion, AFL is common soon after lung transplantation. Those with IPF, advanced age, or left atrial enlargement are at increased risk. In most cases, AFL is a self-limited arrhythmia that resolves spontaneously with no need for ablation.
Atrial arrhythmias are common early in the recovery period after cardiovascular operations. Although atrial flutter (AFL) is known to occur after cardiothoracic surgery, little is known of the epidemiology, risk factors, and presentation of AFL in adult patients who have undergone lung transplantation. AFL is defined as a macro–re-entrant tachycardia involving normal atrial structures and/or atrial scar. Patients with lung transplantation typically have the graft anastomosed to the left atrium. Suture lines in the left atrium can provide electrical discontinuity leading to formation of re-entrant loops. Previous studies investigating atrial arrhythmias after lung transplantation have focused mainly on atrial fibrillation. Those that investigated specifically AFL after lung transplantation have been limited to the pediatric population. To date, no previous study has examined AFL specifically after lung transplantation in adults to determine the incidence, presentation, characteristics, and clinical predictors of AFL.
Methods
We retrospectively reviewed records of all patients undergoing lung transplantation at the University of California, San Francisco, from April 1998 through June 2010. Patients with uni- or bilateral lung transplantation were included in the study.
Patients’ demographic and medical characteristics including age, gender, preoperation diagnosis, and date of transplantation were identified from their hospital records. A 12-lead electrocardiogram (ECG) was obtained before surgery. Patients underwent transesophageal echocardiography before surgery. A standard transplantation technique was used in all patients. Clinical data after transplantation were reviewed to identify postoperative AFL.
All patients underwent continuous telemetry monitoring while in the hospital. Twelve-lead ECGs were obtained for any sustained atrial arrhythmias. ECGs were obtained during outpatient follow-up visits if patients exhibited tachycardia or complained of palpitations. AFL was diagnosed based on review of all 12-lead ECGs obtained after surgery. ECGs were reviewed by 2 electrophysiologists blinded to each other’s diagnosis. ECGs showing AFL were classified as typical or atypical. Typical AFL was defined as positive flutter waves in lead V 1 with negative flutter waves in the inferior leads or negative flutter waves in lead V 1 with positive flutter waves in the inferior leads. All other variations were considered atypical AFL.
Chi-square test was used to compare categorical data. One-way variance analysis was used for continuous variables. Multivariable logistic regression analyses were applied to determine AFL risk factors. Potential confounders were those variables suggested by the literature and those with a p value <0.2 in univariate analysis. The first multivariate analysis included all potential confounders. Variables were removed sequentially until only those variables with p values <0.05 remained in the second multivariate analysis. SPSS 16 for Windows (SPSS, Inc., Chicago, Illinois) was used for analyses. A p value <0.05 was considered statistically significant.
Results
Two hundred sixty-nine patients 15 to 76 years of age who underwent lung transplantation were included for study. These patients were followed serially for the rest of their lives. Baseline characteristics of patients at time of transplantation are listed in Table 1 . Preoperative echocardiograms of the study population revealed right ventricular hypertrophy in 59 (22%), right atrial enlargement in 78 (29%), and left atrial enlargement in 27 (10%). Eighty-two percent (n = 222) underwent bilateral lung transplantation surgery. Idiopathic pulmonary fibrosis (IPF) was the most common indication for lung transplantation (37.9%).
| Demographic characteristics | |
| Age (years) | 53.3 ± 12.9 |
| Men | 150 (55.8%) |
| Cause of lung disease | |
| Idiopathic pulmonary fibrosis | 102 (37.9%) |
| Secondary pulmonary fibrosis | 20 (7.4%) |
| Emphysema | 54 (20.1%) |
| Cystic fibrosis | 25 (9.3%) |
| Primary pulmonary hypertension | 12 (4.5%) |
| Secondary pulmonary hypertension | 12 (4.5%) |
| α-1–antitrypsin deficiency | 10 (3.7%) |
| Hypersensitivity pneumonitis | 8 (2.9%) |
| Lymphangioleiomyomatosis | 7 (2.6%) |
| Others | 19 (7.1%) |
| Pretransplantation echocardiography | |
| Left ventricular hypertrophy | 24 (8.9%) |
| Right ventricular hypertrophy | 59 (21.9%) |
| Left atrial enlargement | 27 (10.0%) |
| Right atrial enlargement | 78 (29.0%) |
| Transplant type | |
| Bilateral | 222 (82.5%) |
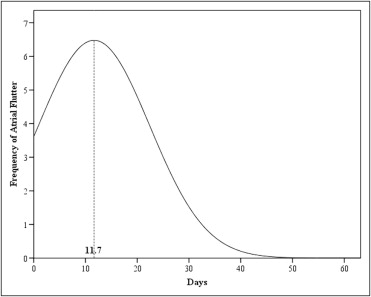
Thirty-five patients (13%) developed AFL. AFL was first documented a mean of 11.7 days after transplantation (range 1 to 51; Figure 1 ). Mean age in the AFL group was 59.9 ± 9.2 years. Patients with AFL were older than those without AFL ( Table 2 ). All patients who developed AFL had no previous atrial arrhythmias. Incidence of atrial fibrillation was also recorded in our patients: atrial fibrillation occurred in 69 patients (26%) over a mean of 7 days (range 1 to 35) after surgery.
| Variable | Atrial Flutter | OR (95% CI) | |
|---|---|---|---|
| No | Yes | ||
| (n = 234) | (n = 35) | ||
| Demographic characteristics | |||
| Age (years), mean ± SD | 52.3 ± 13.1 | 59.9 ± 9.2 | 1.06 (1.02–1.10) ⁎ |
| Men | 53.0% | 74.3% | 2.56 (1.15–5.71) ⁎ |
| Cause of lung disease | |||
| Idiopathic pulmonary fibrosis | 33.8% | 65.7% | 3.76 (1.78–7.95) ⁎ |
| Secondary pulmonary fibrosis | 7.7% | 5.7% | 0.73 (0.16–3.28) |
| Emphysema | 20.9% | 14.2% | 0.63 (0.23–1.71) |
| Cystic fibrosis | 9.8% | 5.7% | 0.56 (0.13–2.47) |
| Primary pulmonary hypertension | 4.7% | 2.9% | 0.59 (0.08–4.77) |
| Secondary pulmonary hypertension | 4.7% | 2.9% | 1.59 (0.08–4.77) |
| α-1–antitrypsin deficiency | 4.3% | 0.0% | 0.96 (0.93–0.98) |
| Hypersensitivity pneumonitis | 3.4% | 0.0% | 0.97 (0.94–0.99) |
| Lymphangioleiomyomatosis | 3.0% | 0.0% | 0.97 (0.95–0.99) |
| Others | 7.7% | 2.9% | 0.35 (0.05–2.73) |
| Pretransplantation echocardiography | |||
| Left ventricular hypertrophy | 8.7% | 17.6% | 2.25 (0.82–6.15) |
| Right ventricular hypertrophy | 26.3% | 14.7% | 0.48 (0.18–1.31) |
| Left atrial enlargement | 9.4% | 23.5% | 2.98 (1.18–7.49) ⁎ |
| Right atrial enlargement | 32.8% | 35.3% | 1.12 (0.52–2.39) |
| Transplant characteristic | |||
| Date (year), mean ± SD | 2,006 ± 2.6 | 2,006 ± 2.6 | 1.02 (0.90–1.17) |
| Bilateral lung transplant | 84.6% | 68.6% | 0.40 (0.18–0.88) ⁎ |
Of the 35 patients who developed AFL, 24 (69%) had atypical AFL. There was no significant difference between typical and atypical AFL groups with regard to demographic and medical characteristics. Of those with atypical AFL, 17 (71%) had undergone bilateral lung transplantation with separate left and right pulmonary vein/left atrial anastomoses, and 7 (29%) had undergone single-lung transplantation. Of those with atypical AFL, 16 (67%) had undergone transplantation because of pulmonary fibrosis.
In univariate analysis, IPF, advanced age, male gender, left atrial enlargement, and bilateral lung transplantation were identified as risk factors for AFL. However, in the first multivariate analysis controlling for all potential confounders ( Table 3 ), only IPF was an independent predictor of AFL (odds ratio 2.6, 95% confidence interval 1.1 to 6.1, p = 0.03). In the second multivariate analysis that included only those variables that were significant at the 0.05 level, advanced age, IPF, and left atrial enlargement were found to be independent risk factors for AFL ( Table 3 ).
| Characteristics | First Multivariate Analysis | Second Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 1.04 (0.99–1.08) | 0.098 | 1.04 (1.01–1.08) | 0.044 |
| Male gender | 1.91 (0.78–4.65) | 0.157 | — | — |
| Idiopathic pulmonary fibrosis | 2.58 (1.08–6.13) | 0.032 | 2.94 (1.31–6.62) | 0.009 |
| Left atrial enlargement | 2.33 (0.84–6.45) | 0.105 | 2.86 (1.07–7.65) | 0.036 |
| Left ventricular hypertrophy | 1.97 (0.65–5.93) | 0.230 | — | — |
| Bilateral lung transplantation | 0.73 (0.29–1.84) | 0.502 | — | — |
Interventional treatment for AFL including electrical cardioversion and radiofrequency catheter ablation was performed in 6 of 35 patients (17%) with AFL. In 5 of these patients (83%), IPF was the indication for lung transplantation. Patients with IPF were 10.3 times as likely to need interventional treatment as those without IPF (p = 0.03).
Electrical cardioversion was performed in 4 patients: 1 (25%) with atypical AFL and 3 (75%) with typical AFL. After cardioversion to sinus rhythm, 3 patients (75%) had no recurrence, whereas 1 patient had recurrence 4 months later and was referred for electrophysiologic study and catheter ablation.
Overall 3 patients were referred for electrophysiologic study and ablation. The first patient had typical counterclockwise AFL. This patient underwent cavotricuspid–isthmus ablation 1 year after lung transplantation and has had no recurrence over 1-month follow-up. The second patient presented with atypical AFL and underwent electrophysiologic study and ablation 28 days after surgery because of rapid ventricular rates despite maximum atrioventricular nodal blockade. He was found to have re-entrant tachycardia between the left upper pulmonary vein and the left atrial appendage. The successful ablation site involved complex, continuous, fractionated signals spanning flutter diastole, judged to be at or near the suture line between grafted and recipient left atria. Ablation at this site terminated the tachycardia and the patient had no recurrence of tachycardia after 6-month follow-up. The third patient initially developed atypical AFL 4 days after transplantation. He ultimately underwent radiofrequency ablation 18 months after surgery because of persistence of the arrhythmia, and a re-entrant left AFL was mapped to an area of scar adjacent to the left upper pulmonary vein. Radiofrequency was performed above and posterior to the left upper pulmonary vein, resulting in termination of the flutter. The flutter could not be reinduced with pacing maneuvers. He had recurrent atypical flutter 8 months later and underwent a second ablation. A macro–re-entrant left AFL arising from an area of slow conduction between the left pulmonary veins apparently near the left pulmonary vein cuff anastomosis was observed ( Figure 2 ). A linear lesion was created from the left upper pulmonary vein to the left lower pulmonary vein, which successfully terminated the arrhythmia.




