Peripheral and central pulmonary artery (PA) stenoses can result in right ventricular hypertension, dysfunction, and death. Percutaneous PA angioplasty and stent placement relieve obstruction acutely, but patients frequently require reintervention. Within a heterogeneous patient population with PA stents referred for catheterization because of noninvasive signs of PA obstruction, we have observed that in-stent stenosis (ISS) occurs commonly in some groups, challenging previous reports that this phenomenon occurs infrequently. We set out to evaluate the incidence and demographics of patients with previous PA stent placement who develop ISS. Consecutive patients with previously placed stents presenting for catheterization and undergoing PA angiography were reviewed (104 patients, 124 cases). We defined ISS angiographically, as a 25% narrowing of the contrast-filled lumen relative to the fluoroscopically apparent stent diameter at any site along the length of the stent. For inclusion, we required that the stenotic segment be narrower or equal in size to the distal vessel. ISS was diagnosed in 24% of patients, with the highest incidence among patients with tetralogy of Fallot and multiple aortopulmonary collaterals, Williams syndrome, or Alagille syndrome. In conclusion, ISS after PA stent placement is a more frequent problem than previously reported, and patients with inherently abnormal PAs are disproportionately affected. Increased clinical surveillance after stent placement and investigation of innovative preventive strategies may be indicated.
Peripheral pulmonary stenosis (PPS) occurs in the context of diffuse arteriopathies (e.g., Williams syndrome) and cardiac developmental defects (e.g., tetralogy of Fallot [TOF]). In either case, multiple lobar and/or sublobar obstructions can lead to proximal pulmonary artery (PA) hypertension. Although right ventricular (RV) function is typically preserved, this can result in severe RV hypertension. Unmitigated, this RV hypertension results in arrhythmia, ventricular failure, and death. PPS is managed surgically when lesions are located proximally, but more peripheral disease usually calls for endovascular management, with transcatheter balloon angioplasty or stent placement. Stents are known to be effective in lesions refractory to balloon angioplasty and have greatly enhanced the therapeutic armamentarium of the interventionalists. Rates of in-stent stenosis (ISS) have previously been reported to be very low. However, among patients with PPS referred for catheterization, we have observed a significant rate of angiographic ISS. Currently, no published information is available on rates of ISS among patients with PPS. We thus set out to describe the incidence of ISS in this patient cohort.
Methods
The aim of this study was to determine the incidence of ISS in (1) patients with PPS referred for catheterization and (2) patients with Williams or Alagille syndrome. To address the first objective, we searched an institutional database for consecutive patients having catheterization during the year 2012 who had undergone PA stent placement within the previous 10 years. This list was supplemented with any patient who had a PA stent redilated at our institution during the year 2012; this allowed us to identify some additional patients whose stents were not initially placed at our institution or placed >10 years ago. Cases were excluded from consideration if there were no angiographic data available for review or if the stent had fractured in a manner precluding meaningful measurement of stent or luminal diameter. A total of 124 cases (104 patients) were included in our review. Indication for catheterization in this patient population at our institution has remained consistent for many years, including preoperative examination, noninvasive imaging suggestive of significantly elevated RV pressure (≥2/3 of systemic), pulmonary blood flow maldistribution (<1/3 of flow to 1 lung), worsening RV function, cyanosis due to PA stenosis, or worsening in symptoms.
To address the second objective, we searched the institutional database to identify all patients with either Williams or Alagille syndrome who had been catheterized at our center at any time after PA stent placement. This identified 14 patients with Williams syndrome and 12 patients with Alagille syndrome who had undergone angiographic evaluation subsequent to PA stent placement at our center from 2003 to 2012. The diagnosis of either syndrome was based on documentation of meeting genetic and/or clinical criteria.
We defined ISS angiographically, as a 25% narrowing of the contrast-filled lumen relative to the fluoroscopically apparent stent diameter at any site along the length of the stent or stent complex ( Figure 1 ). In addition, the segment was only deemed stenotic if it was equal to or narrower than the distal vessel to avoid inclusion of ISS due to significant stent overexpansion at the time of placement. Twenty-five percent ISS was empirically chosen based on our observation that this degree of angiographic narrowing is often hemodynamically significant and responsive to redilation. Determination of ISS was expressed as percentage of current stent diameter, as opposed to absolute measurement of luminal compartments. This allowed us to accommodate for the large range in patient and stent sizes in the pediatric population. Furthermore, defining ISS by percentage of stent lumen (rather than by a certain number of mm) removed much of the risk of calibration error, which otherwise could be significant, particularly in small vessels.
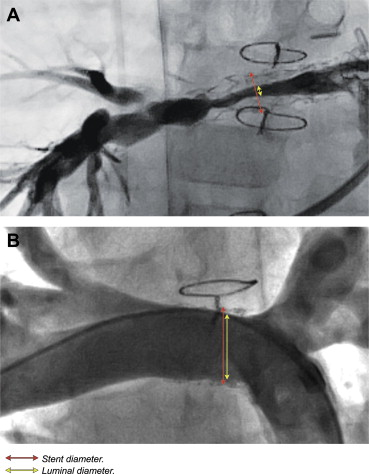
Data collection was performed by evaluation of 2012 angiographic records for assessment of ISS and review of medical records and a cardiology department clinical database for demographic information. In general, images were acquired in 2 planes, and the best longitudinal image of each stent was evaluated. Among patients with multiple PA stents, the identification of ISS in any of the stents resulted in the patient being considered as affected. In these cases, measurements were performed on only one of the stents, typically the one that was most severely narrowed for purposes of data analysis. Computerized 2-dimensional measurements were performed using Vericis 8.30 (Merge Healthcare, Chicago, Illinois) by measuring the narrowest contrast-filled lumen diameter inside the stent and the fluoroscopically apparent stent diameter at that site. Values are expressed as median with a range or means with SD where applicable. ISS rates in different diagnostic groups were compared using Fisher’s exact test. Two-tailed paired t tests were used to compare means of continuous variables. Statistical significance was set at p < 0.05.
Results
Age at the time of catheterization in 2012 was 5.5 years (range, 0.1 to 64). As expected based on our practice, approximately 40% of cases were performed on patients with TOF, pulmonary atresia, and multiple aortopulmonary collaterals (TOF/pulmonary atresia/MAPCAs). Other forms of biventricular conditions, including D-loop transposition of the great arteries (D-TGA) and truncus arteriosus, made up 19% of cases. Single ventricle conditions, primarily hypoplastic left heart syndrome (HLHS) (at any stage of palliation), made up 15% of cases. Patients with PPS from an identified arteriopathy made up 8% of total cases. Total lifetime catheterizations through 2012 ranged from 2 to 19 (median 6). Time since stent placement was 2.8 years (range 0.04 to 19) and time since most recent stent dilation was 1.5 years (range 0.03 to 13). Approximately 3/4 of stents were Genesis (Cordis, Miami Lakes, Florida) stents (premounted or XD). Demographics of patients evaluated to address the first objective are listed in Table 1 . There were 26 patients (Williams syndrome n = 14, Alagille syndrome n = 12) in the group addressing the second objective. Age at the time of catheterization in this group was 4.4 years (range 0.5 to 20).
| Variable | All Cases (n = 124) |
|---|---|
| Male | 56% |
| Age at catheterization (yrs) | 5.5 (0.10–64) |
| Body surface area at catheterization (m 2 ) | 0.88 ± 0.47 |
| Weight at catheterization (kg) | 26.6 ± 22 |
| Stent redilation performed (% of cases) | 80 |
| Total catheterizations per patient (n) | 6 (2–19) |
| PA stents per patient | 1 (1–7) |
| Time since stent placement (yrs) | 2.8 (0.04–19) |
| Time since the most recent stent dilation (yrs) | 1.5 (0.03–13) |
| Diagnosis | |
| TOF with MAPCAs | 48 (39) |
| TOF without MAPCAs | 16 (13) |
| MAPCAs (not TOF) | 6 (4.8) |
| Alagille syndrome ∗ | 7 (5.6) |
| Williams syndrome | 4 (3.2) |
| Nonsyndromic PPS | 5 (4.0) |
| Truncus arteriosus | 5 (4.0) |
| D-TGA | 6 (4.8) |
| Other biventricular | 13 (11) |
| HLHS | 15 (12) |
| Other univentricular | 5 (4.0) |
| Stent type | n = 133 stents |
| Genesis premounted | 76 (57) |
| Genesis XD | 21 (16) |
| Coronary | 6 (5) |
| Other | 15 (11) |
| Unavailable | 15 (11) |
∗ Of 7 cases with Alagille syndrome, 6 had TOF (also accounted for in their respective TOF group).
In this patient cohort, the incidence of angiographic PA ISS was 24% (25 of 104 patients), representing 124 total cases. In affected patients (i.e., by our definition ≥25% ISS and at most equal luminal size to distal vessel), the average degree of ISS was 43% (SD 12%, range 27% to 72%) stenosis, that is, on average, the in-stent lumen was narrowed by nearly half. Subgroup analysis of ISS severity was not performed because of limited sample size of affected patients. Mean time since stent placement was similar for patients with and without ISS (4.1 ± 4.1 and 4.2 ± 4.1 years, respectively). Time since last intervention was less in patients with ISS (1.5 ± 1.6 years) compared with those without significant ISS (2.7 ± 2.9 years, p <0.05). The distribution of stent types was also similar between the 2 groups (p >0.05). Stent redilation was performed in 80% of total cases (100% of cases with ISS). Presumably, redilations without underlying ISS were performed to accommodate somatic growth.
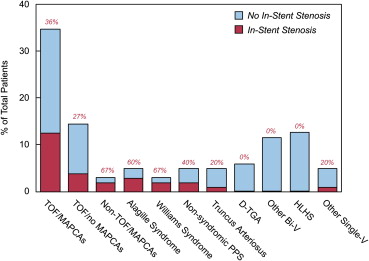
Some patient groups had a high tendency to be affected by ISS, whereas others were rarely affected ( Figure 2 ). Patients with MAPCAs had a 38% incidence of ISS, specifically 13 (36%) of 36 patients with TOF/pulmonary atresia/MAPCAs and 2 of 3 patients with other conditions associated with MAPCAs. Underlying diagnoses of the latter 2 patients were complex biventricular heterotaxy with pulmonary atresia and single RV with pulmonary atresia, respectively. The average severity of ISS among affected patients in the TOF/pulmonary atresia/MAPCA group was 44%, that is, similar to the average for the entire cohort affected by ISS. In patients with TOF without MAPCAs, the incidence was 27% (4 of 15 patients). There were 7 patients with 22q11 deletion in the cohort, all of whom had TOF/pulmonary atresia/MAPCAs but interestingly had a low incidence (14%) of ISS (1 of 7 patients), which was not significantly different from the rate seen in TOF/pulmonary atresia/MAPCA patients without the 22q11 deletion based on the present small sample size. ISS was nearly absent in other structural congenital heart disease, including HLHS at any stage of repair (0 of 13 patients), D-TGA (0 of 6 patients), truncus arteriosus (1 of 5 patients), and other biventricular conditions (0 of 12). This latter group of patients with other structural congenital heart disease was significantly less affected by ISS compared with the patients with TOF and/or MAPCAs or arteriopathy (p <0.01; Figure 3 ). The more extensive review of patients with genetic arteriopathic conditions supported their high incidence of ISS, including 36% of patients (5 of 14) with Williams syndrome and 50% of patients (6 of 12) with Alagille syndrome (9 of whom also had TOF).