Excess dosing of anticoagulant agents has been linked to increased risk of bleeding after percutaneous coronary intervention (PCI) for women compared with men, but these studies have largely included older patients. We sought to determine the prevalence and gender-based differences of excess dosing of anticoagulants including glycoprotein IIb/IIIa inhibitors, bivalirudin, and unfractionated heparin in young patients with acute myocardial infarction who underwent PCI and to examine its association with bleeding. Of 2,076 patients enrolled in the Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients study who underwent PCI, we abstracted doses of unfractionated heparin, bivalirudin, and glycoprotein IIb/IIIa inhibitors administered during PCI from the medical records. At least 47.2% received at least 1 excess dose of an anticoagulant, which did not differ by gender. We used logistic regression to determine the predictors of excess dosing and the association of excess dosing with bleeding. In multivariable analysis, only lower body weight and younger age were significant predictors of excess dosing. Bleeding was higher in young women who received excess dosing versus those who did not (9.3% vs 6.0%, p = 0.03) but was comparable among men (5.2% vs 5.9%, p = 0.69) in univariate analysis. In multivariable analysis, there was a trend to an association between excess dosing and bleeding (odds ratio 1.33, 95% confidence interval 0.92 to 1.91) although not statistically significant. In conclusion, approximately half of the patients received excess dosing of anticoagulant drugs during PCI, which did not vary based on gender. There was a trend toward an association between excess dosing and increased bleeding, although not statistically significant.
Anticoagulants and antiplatelet agents are commonly used in the management of patients who undergo percutaneous coronary intervention (PCI) but are prone to dosing errors. Despite weight-based and renal dosing guidelines, anticoagulants continue to be administered in excess of the recommended dose in almost half of the patients with both non–ST-elevation and ST-elevation acute coronary syndromes, including a high proportion of patients undergoing PCI. Dosing errors have been linked to increased risk of bleeding complications after PCI for women compared with men, but these studies largely focused on older patients. To investigate potential dosing errors in younger patients, we analyzed data from the Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients (VIRGO) study that recruited patients with acute myocardial infarction (AMI) who were ≤55 years. Specifically, we identified VIRGO participants who underwent PCI during their initial hospitalization with AMI. In this population, we determined the prevalence and gender-based differences in excess dosing of anticoagulant drugs including, glycoprotein IIb/IIIa inhibitors(GPIs) (eptifibatide and abciximab), bivalirudin, and unfractionated heparin (UFH). We also examined the association between excess dosing of these drugs and in-hospital bleeding events.
Methods
From August 8, 2008, to January 5, 2012, a total of 3,572 patients with AMI from 103 US hospitals, 24 Spanish hospitals, and 3 Australian hospitals were enrolled in the VIRGO study. The study used a 2:1 female to male enrollment design to enrich the study’s inclusion of previously understudied young women. We limited our study to the 2,985 patients enrolled from the United States only. The methods of VIRGO have been described previously. In brief, participants were 18 to 55 years old, and AMI was confirmed by increased cardiac biomarkers within 24 hours of admission and with either ischemic symptoms or electrocardiographic evidence of AMI. Participants must have presented directly to the enrolling site, or been transferred within 24 hours of presentation, thus ensuring that primary clinical decision making occurred at the enrolling site. Exclusion criteria included (1) non–English and/or non–Spanish-speaking patients, (2) inability to provide informed consent, (3) incarceration, and (4) those patients who developed elevated cardiac markers as a result of elective coronary revascularization. Institutional review board approval was obtained at each participating institution, and patients provided informed consent for their study participation including baseline and follow-up interviews.
Of 2,985 patients enrolled from the United States, 2,172 patients underwent PCI during their initial hospitalization in the study cohort and were included in our study. Of these, we excluded patients with missing information regarding creatinine and weight, which was necessary to calculate renal- or weight-based drug dosing, leading to a final population of n = 2,076. For patients who underwent PCI more than once, we only considered information from the initial PCI. We abstracted data for the administration of GPIs, bivalirudin, and UFH administered before and during PCI from the patients’ medical records. For each medication, recommended dosing was defined in accordance with the AHA guidelines, package inserts, and clinical trial publications. We characterized excess dosing into minor and major categories ( Table 1 ). Minor excess dosing was defined as administration of the drug in up to 10% excess of the recommended dose. Major excess dosing was defined as administration of the drug in >10% excess of the recommended dose.
| Drug | Recommendations during PCI | Minor Excess Dosing | Major Excess Dosing |
|---|---|---|---|
| GPIs | |||
| Abciximab | Bolus 0.25 mg/kg IV; Infusion 0.125 mcg/kg/min (maximum 10 mcg/min) | 0.25mg/kg> Bolus <0.275 mg/kg; 0.125 mcg/kg/min > Infusion <0.1375 mcg/kg/min | Bolus > 0.275 mg/kg; Infusion >0.1375 mcg/kg/min OR Infusion > 10 mcg/min whichever is less |
| Eptifibatide | Bolus 180 mcg/kg IV; Infusion 2.0 mcg/kg/min, reduce to 1.0 mcg/kg/min if CrCl<50 mL/min | 180 mcg/kg > Bolus < 198 mcg/kg; 2.0 mcg/kg/min > Infusion rate < 2.2 mcg/kg/min | Bolus > 198 mcg/kg; Infusion rate > 2.2 mcg/kg/min OR; Full dose if CrCl< 50 mL/min |
| Bivalirudin | Bolus 0.75 mg/kg; Infusion 1.75 mg/kg/hour | 0.75 mg/kg > Bolus < 0.825 mg/kg; 1.75 mg/kg/hour > Infusion rate < 1.925 mg/kg/hour | Bolus > 0.825 mg/kg; Infusion rate > 1.925 mg/kg/hour |
| Unfractionated Heparin | With GPIs : 50-70 U/kg No GPIs: 70-100 U/kg; Infusion rate: 12-15 U/kg/hour | With GPIs: 70U kg >UFH bolus<77 U/kg ; No GPIs: 100 U/kg> UFH bolus< 110 U/kg; 15 U/kg/hour> Infusion <16.5 U/kg/hour | With GPIs : UFH bolus> 77 U/kg; No GPIs: UFH bolus> 110 U/kg; Infusion>16.5 U/kg/hour |
For GPIs, the strength of the solution administered (eptifibatide 2 mg/ml or 0.75 mg/ml; abciximab 2 mg/ml or 9 mg/250 ml or other) was recorded to determine the dose of bolus (eptifibatide [μg/kg] and abciximab [mg/kg]) and infusion (μg/kg/min for both). For bivalirudin, the bolus (mg or ml) and infusion (mg/h or ml/h) doses as recorded in the medical record abstraction tool were converted into the bolus (mg/kg) and infusion (mg/kg/h) doses administered using strength of bivalirudin (5 mg/ml) and total body weight. For UFH, bolus (U) and infusion doses (U/h) were divided by total body weight (kg) to determine the doses in units per kilogram and units per kilogram per hour, respectively. For UFH boluses, we classified patients as receiving excess dosing if any individual bolus, not cumulative dose, was in >10% excess of standard dosing guidelines.
We estimated glomerular filtration rate (GFR) using the Chronic Kidney Disease Epidemiology Collaboration equation that has been shown to estimate GFR with the highest precision and accuracy, particularly in the younger age groups and in women. We defined patients as having renal dysfunction if their estimated GFR (eGFR) value was ≤60 ml/min/1.73 m 2 .
We defined bleeding as (1) occurring at percutaneous entry site, during or after catheterization laboratory visit until discharge, which may be external or a hematoma >10 cm for femoral, >5 cm for brachial, or >2 cm for radial access; (2) retroperitoneal; (3) gastrointestinal; (4) genitourinary; and (5) other and/or unknown origin during or after catheterization laboratory visit until discharge. All bleeding events required a transfusion, prolonged hospital stay, and/or a decrease in hemoglobin >3.0 g/dl. This was in accordance with the commonly used definitions (CathPCI registry).
Patient characteristics, presentation, and treatment are shown for overall patients and are stratified by gender and whether they received excess dosing of anticoagulants. Categorical variables are reported as numbers (percentages), and continuous variables are reported as medians (interquartile ranges) or mean (SD). We tested the significance of observed differences with the chi-square tests for categorical variables and Wilcoxon rank-sum tests or t tests for continuous variables. We explored factors associated with excess dosing using multivariable logistic regression to adjust for patient demographics and co-morbidities previously shown to be associated with increased risk of both excess dosing and bleeding.
We explored factors associated with bleeding in patients receiving these therapies using multivariable logistic regression and adjusted for excess dosing and other sociodemographic and clinical covariates previously shown to affect risk of bleeding. These included gender, age, weight, eGFR, diabetes mellitus, PCI urgency, systolic blood pressure, and previous peripheral arterial disease. We repeated the analysis to examine the effect of “major” excess dosing in place of “any” excess dosing on bleeding and repeated the bleeding models including a gender-excess dosing interaction. We considered a 2-sided p <0.05 as statistically significant. All analyses were done using SAS 9.3 (SAS Institute Inc., Cary, North Carolina) and VIRGO data, version 1.0.
Results
The final cohort consisted of 2,076 patients with AMI who underwent PCI, 1,311 women and 765 men. A total of 1,172 patients (56.5%) received GPIs, 692 patients (33.3%) received bivalirudin and 1,623 patients (78.2%) received UFH. Of these, 352 patients received a combination of bivalirudin and UFH, 152 patients received a combination of bivalirudin and GPIs, and 982 patients received a combination of UFH and GPIs.
Although both women and men were of similar age, women had a lower mean body weight than men (86.7 vs 97.8 kg; p <0.01; Table 2 ). Overall, 47.2% of the patients received at least 1 dose of an anticoagulant in excess, and 6.6% of patients received >1 class of medications in excess. Within individual categories of medications, 40% of the patients receiving GPIs, 49% of the patients receiving bivalirudin, and 18.9% of the patients receiving UFH received at least 1 excess dose of these drugs ( Table 3 ).
| Patient characteristics | Overall (n=2076) | Women (n=1311) | Men (n=765) | P value |
|---|---|---|---|---|
| Age (years), median (IQR) | 48.0 (44.0 – 52.0) | 49.0 (44.0 – 52.0) | 48.0 (44.0 – 52.0) | 0.13 |
| Obesity (BMI ≥ 30 kg/m 2 ) | 1133 (54.6%) | 764 (58.3%) | 369 (48.2%) | <0.01 |
| Weight (kg), mean (std) | 90.8 (23.1) | 86.7 (22.6) | 97.8 (22.2) | <0.01 |
| Nonwhite race | 469 (22.6%) | 335 (25.6%) | 134 (17.5%) | <0.01 |
| Diabetes mellitus | 720 (34.7%) | 532 (40.6%) | 188 (24.6%) | <0.01 |
| Hypertension | 1368 (65.9%) | 885 (67.5%) | 483 (63.1%) | 0.04 |
| Current smokers | 1194 (57.5%) | 788 (60.1%) | 406 (53.1%) | <0.01 |
| Prior stroke | 84 (4.0%) | 69 (5.3%) | 15 (2.0%) | <0.01 |
| Prior myocardial infarction | 319 (15.4%) | 190 (14.5%) | 129 (16.9%) | 0.15 |
| Prior revascularization | 362 (17.5%) | 211 (16.1%) | 151 (19.9%) | 0.03 |
| Prior heart failure | 68 (3.3%) | 58 (4.4%) | 10 (1.3%) | <0.01 |
| eGFR (ml/min/1.73m 2 ), mean (std) | 89.1 (23.1) | 88.9 (24.7) | 89.4 (20.2) | 0.64 |
| eGFR<50 ml/min/1.73m 2 | 98 (4.7%) | 80 (6.1%) | 18 (2.4%) | <0.01 |
| Renal Dysfunction (eGFR<=60) | 211 (10.2%) | 151 (11.6%) | 60 (7.9%) | 0.01 |
| Serum Creatinine (mg/dL), median (IQR) | 0.9 (0.7 – 1.0) | 0.8 (0.7 – 0.9) | 1.0 (0.9 – 1.1) | <0.01 |
| Creatinine >2mg/dL | 45 (2.2%) | 33 (2.5%) | 12 (1.6%) | 0.15 |
| Presentation variables | ||||
| Signs of heart failure | 69 (3.5%) | 51 (4.1%) | 18 (2.5%) | 0.06 |
| Systolic blood pressure (mm Hg), mean (std) | 144.7 (31.0) | 143.5 (32.4) | 146.7 (28.4) | 0.02 |
| Heart rate (bpm), mean (std) | 82.8 (19.6) | 83.6 (19.2) | 81.4 (20.1) | 0.02 |
| ST depression | 855 (41.5%) | 544 (41.8%) | 311 (40.9%) | 0.66 |
| Treatment | ||||
| Aspirin | 2012 (97.3%) | 1263 (96.9%) | 749 (98.2%) | 0.07 |
| Unfractionated heparin | 1623 (78.2%) | 1009 (77.0%) | 614 (80.3%) | 0.08 |
| Glycoprotein IIb/IIIa inhibitors | 1172 (56.5%) | 717 (54.7%) | 455 (59.5%) | 0.03 |
| Eptifibatide | 899 (43.3%) | 556 (42.4%) | 343 (44.8%) | 0.28 |
| Abciximab | 273 (13.2%) | 161 (12.3%) | 112 (14.6%) | 0.12 |
| Clopidogrel | 1718 (89.2%) | 1080 (89.5%) | 638 (88.6%) | 0.55 |
| Prasugrel | 256 (13.3%) | 156 (12.9%) | 100 (13.9%) | 0.55 |
| Ticlopidine | 3 (0.2%) | 2 (0.2%) | 1 (0.1%) | 1.00 |
| Bivalirudin | 692 (33.3%) | 459 (35.0%) | 233 (30.5%) | 0.03 |
| Drugs | Any excess dosing (>0%) | Minor excess dosing (0-10%) | Major excess dosing (>10%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | P value | Female | Male | P value | Female | Male | P value | |
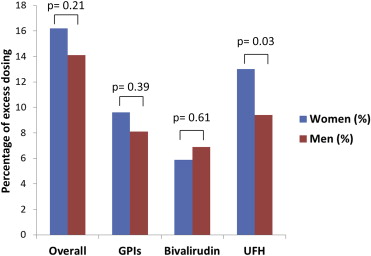
| Overall | 621 (47.4%) | 359 (46.9%) | 0.85 | 409 (31.2%) | 251 (32.8%) | 0.45 | 212 (16.2%) | 108 (14.1%) | 0.21 |
| GPIs | 287 (40.0%) | 187 (41.1%) | 0.72 | 218 (30.4%) | 150 (33.0%) | 0.36 | 69 (9.6%) | 37 (8.1%) | 0.39 |
| Bivalirudin | 220 (47.9%) | 119 (51.1%) | 0.43 | 193 (20.4%) | 103 (19.7%) | 0.75 | 27 (5.9%) | 16 (6.9%) | 0.61 |
| UFH | 206 (20.4%) | 100 (16.3%) | 0.04 | 75 (10.0%) | 42 (8.5%) | 0.37 | 131 (13.0%) | 58 (9.4%) | 0.03 |
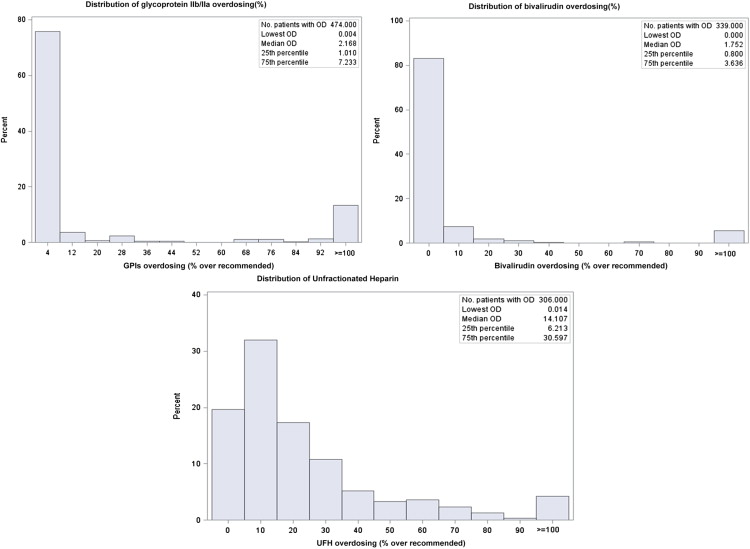
Of note, 25.9% (n = 51) of the patients who were administered excess UFH boluses received a dose of exactly 5,000 units and 21.9% (n = 30) of the patients who were infused UFH in excess received a dose of exactly 1,000 U/h when heparin therapy was initiated. For GPIs and bivalirudin, excess dosing was most frequently minor (77.6% and 87.3%, respectively). However, 61.8% of excess dosing for UFH was major ( Figure 1 ). Overall, the proportion of women who received at least 1 excess dose of these anticoagulant agents was not significantly different from men ( Figure 2 ). However, UFH was administered in excess more frequently in women (n = 206 [20.4%]) compared with men (n = 100 [16.3%]; p value = 0.04; Table 3 ).
In univariate analyses, patients receiving excess dosing were less likely to be obese (49.9% vs 58.8%; p <0.0001) or to have diabetes (32% vs 37%; p = 0.02) than patients who did not receive excess dosing. In multivariate logistic analysis, female gender was not significantly associated with excess dosing (OR 0.89, 95% CI 0.73 to 1.08). Both lower body weight (OR 1.07 per 5 kg decrease in weight, 95% CI 1.05 to 1.09) and younger age (OR 0.93 per 5 years increase in age, 95% CI 0.86 to 0.99) were significantly associated with increased likelihood of excess dosing ( Table 4 ). In secondary analyses, we performed multivariate logistic analysis to identify predictors of major excess dosing, and the findings did not differ significantly from any excess dosing.



