Vascular access and closure remain a challenge in transcatheter aortic valve replacement (TAVR). This single-center study aimed to report the incidence, predictive factors, and clinical outcomes of access-related vascular injury and subsequent vascular intervention. During a 30-month period, 365 patients underwent TAVR and 333 patients (94%) were treated by true percutaneous transfemoral approach. Of this latter group, 83 patients (25%) had an access-related vascular injury that was managed by the use of a covered self-expanding stent (n = 49), balloon angioplasty (n = 33), or by surgical intervention (n = 1). In 16 patients (5%), the vascular injury was classified as a major vascular complication. Absence of a preprocedural computed tomography angiography (CTA) of the iliofemoral arteries (OR 2.04, p = 0.007) and female gender (OR 2.18, p = 0.004) were independent predictors of the need for access-related vascular intervention. In addition, a high sheath/common femoral artery ratio as measured on preoperative CTA was associated with a higher rate of post-TAVR vascular intervention. The radiation dose, iodine contrast volume, transfusion need, length of hospitalization, and 30-day mortality were not significantly different between patients with versus without access-related vascular intervention. In conclusion, access-related vascular intervention in patients who underwent transfemoral-TAVR is not uncommon. Female gender and a high sheath/common femoral artery ratio are risk factors for access-related vascular injury, whereas preprocedural planning with CTA of the access vessels may reduce the risk of vascular injury. Importantly, most access-related vascular injuries may be treated by percutaneous techniques with similar clinical outcomes to patients without vascular injuries.
Transcatheter aortic valve replacement (TAVR) has fundamentally altered the treatment strategy for patients deemed at high risk or not eligible for surgical aortic valve replacement. Several approaches are possible, that is, a transfemoral (TF), transsubclavian, transcarotid, direct aortic, and transapical approach. The true percutaneous TF approach is the most widely used ; however, this comes with the challenge of vascular closure device failure and, hence, access-related vascular complications. In contrast, a true percutaneous TF approach has the advantage to avoid a surgical intervention, resulting in a reduced procedural time, blood loss, infection risk, length of hospital stay, and mortality. The aim of this study was to report the incidence, predictive factors, and clinical outcomes of access-related vascular injuries and subsequent vascular intervention in a TAVR center treating the vast majority of its patients by true percutaneous TF approach.
Methods
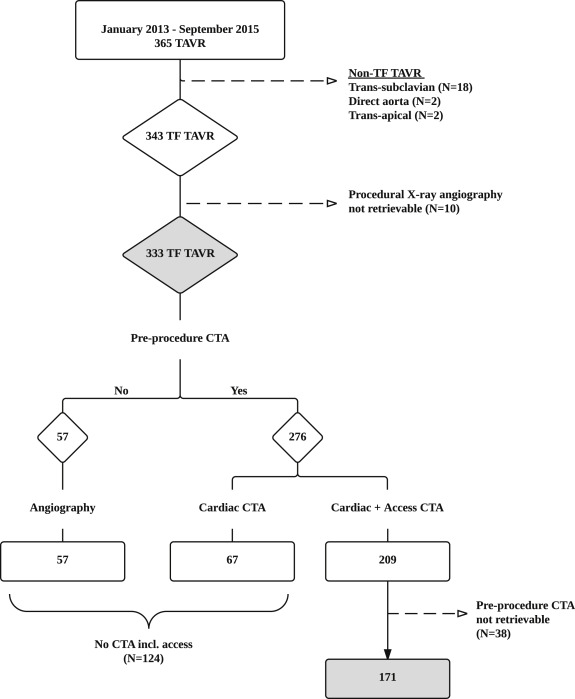
All patients who underwent TAVR at Rigshospitalet University Hospital, Copenhagen, Denmark, from January 2013 to September 2015 were considered for this study. All TAVR patients who underwent TF-TAVR were included in the study population. Patients who had a retrievable preprocedural computed tomography angiography (CTA) of the iliofemoral access vessels were included in a CTA imaging subanalysis ( Figure 1 ). Data were prospectively collected and retrospectively analyzed. An informed consent was obtained from each patient, and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
The preprocedural CTA furnished data on the access vessel size, calcium burden, and tortuositas. The minimal lumen diameter (mm) and area (mm 2 ) of the common femoral artery (CFA) were assessed from inner to inner wall. The outer diameter of the introducer sheath—or delivery system in case of a sheathless procedure—was used to calculate the sheath/CFA ratio, both for diameter and area. The calcium burden was reviewed with an optimized preset window width/level of Hounsfield units. Vessel wall calcium depots at the puncture site were graded as follows: none, grade I (<25% of the circumference), grade II (25% to 50% of the circumference), and grade III (>50% of the circumference)—as evaluated for both the anterior or posterior wall. The calcium volume (mm 3 ) at the puncture site including a 10-mm vessel segment proximal to the puncture site was calculated by manual sculpturing the plaques on a 3-dimensional reconstructive software console. Two operators independently analyzed image data twice in a subset of 40 patients to test repeatability and reproducibility. The analysis was made on a 3-dimensional computer workstation (Vitrea, version 6.2; Vital Images, Minnetonka, Minnesota).
The TAVR procedures were performed as described previously. Pre-closure of the access vessel was done in all cases using a Prostar-XL or Proglide system (Abbott Vascular, Chicago, Illinois). The large bore sheaths and/or delivery systems were inserted to perform the TAVR procedure. At the end of the TAVR procedure, the large bore sheath or delivery system was extracted and the sutures were tied up to ensure hemostasis. A final digital subtraction angiography was made through the contralateral side to evaluate adequate hemostasis and assess vascular patency.
Access-related vascular injuries were defined as vascular rupture, major or minor extravasation, dissection, or stenosis requiring any type of vascular intervention. The following end points were analyzed: total procedure time (minutes), dose-area product (Gycm 2 ), iodine contrast volume (ml), and any unplanned access-related vascular intervention at the end of the procedure. Major vascular complication was defined according to the Valve Academic Research Consortium-2 criteria. Finally, need for transfusion, length of hospitalization stay, and 30-day mortality were also evaluated. Percutaneous vascular interventions were either balloon angioplasty with a Tyshak balloon (NuMED, Hopkinton, New York) or use of a covered self-expanding Viabahn stent (Gore Inc., Flagstaff, Arizona) to achieve hemostasis or correct vessel obstruction.
Categorical variables are reported as absolute values and percentage (%). Continuous variables are expressed as mean ± SD. Categorical variables were compared using the chi-square or Fisher’s exact test and continuous variables using the Student’s t test or the Mann-Whitney test, as appropriate. Univariate logistic regression analysis was used to identify variables associated with access-related vascular intervention. All variables with a p value <0.10 on the univariate analysis were included in a multivariate analysis to identify independent predictors of vascular intervention. Colinearity between sheath/CFA diameter ratio and sheath/CFA area ratio was proved; consequently, no multivariate analysis was performed for the CTA variables. All tests were 2 sided, and a p value <0.05 was considered statistically significant. Repeatability of measurements was assessed in Bland-Altman plots and linear mixed models for repeated measurements to assess the reproducibility. Analyses were performed with SPSS 22.0 software (IBM Corp, Armonk, New York), except repeatability and reproducibility analyses were performed with SAS, version 9.4 (SAS Institute, Cary, North Carolina).
Results
During the study period, a total of 365 TAVR were performed. In total, 22 patients (6%) were treated by an alternative non-TF approach and 343 patients (94%) were treated by TF approach. The procedural x-ray angiography could not be retrieved from 10 patients, resulting in a final study population of 333 patients who underwent TF-TAVR ( Figure 1 ). Baseline patient and procedural characteristics are reported in Table 1 . The femoral access site was closed using a Prostar XL or ProGlide vascular closure device in 314 patients (94%) and 19 patients (6%), respectively.
| Variable | TF-TAVR (n = 333) |
|---|---|
| Age (years) | 80.8 ± 6.7 |
| Male | 173 (52%) |
| Hypertension | 244 (73%) |
| Hypercholesterolemia | 171 (51%) |
| Body mass index, kg/m 2 | 26.9 ± 5.5 |
| Diabetes mellitus | 66 (20%) |
| Current smoker | 13 (4%) |
| Coronary artery disease | 126 (38%) |
| Atrial fibrillation | 85 (26%) |
| Peripheral artery disease | 42 (13%) |
| GFR <60 mL/min/1.73 m2 | 91 (27%) |
| GFR <30 mL/min/1.73 m2 | 7 (2%) |
| Chronic obstructive lung disease | 64 (19%) |
| NYHA ≥ 3 | 211 (63%) |
| Left ventricular ejection fraction | 48.7 ± 12.6 |
| Mean gradient | 35.4 ± 18.4 |
| Aortic valve area | 0.67 ± 0.2 |
| STS surgical risk score | 4.6 ± 1.8 |
| Transcatheter heart valve | |
| CoreValve | 222 (67%) |
| Sapien-3 | 37 (11%) |
| Portico | 31 (9%) |
| Lotus Valve System | 27 (8%) |
| EvolutR | 16 (5%) |
| Closure device | |
| Prostar XL | 314 (94%) |
| Proglide | 19 (6%) |
| Procedure time (min) | 80.2 ± 37.4 |
| Iodine contrast volume (mL) | 107.3 ± 42.0 |
| Fluoroscopy time (min) | 23.6 ± 9.5 |
| Dose area product (Gycm 2 ) | 155.3 ± 143.4 |
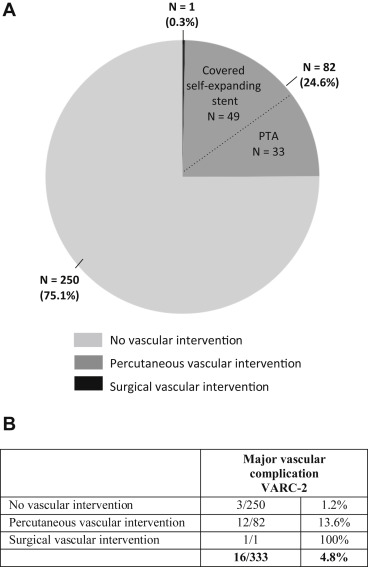
An access-related vascular intervention was judged necessary in 83 of 333 patients (25%). A covered self-expanding Viabahn stent was used in 49 cases because of vascular rupture (n = 9), major extravasation (n = 33), vascular dissection (n = 3), and vascular stenosis (n = 4). In addition, percutaneous transluminal angioplasty with a Tyshak balloon was performed in 33 cases because of major extravasation (n = 27), vascular dissection (n = 1), and arterial stenosis (n = 5). Surgical vascular repair was only needed in 1 patient ( Figure 2 ). As a result, a Valve Academic Research Consortium-2–defined major vascular complication was noted in 16 patients (<5%). Of these, 13 patients underwent “ad hoc” vascular intervention, whereas 3 patients had no obvious vascular injury after percutaneous vascular closure but still developed a hematoma or pseudoaneurysm the day(s) after ( Figure 2 ).
Logistics regression analysis indicates that female gender (OR 2.18, p = 0.004) and absence of preprocedural CTA imaging of the access vessels (OR 2.04, p = 0.007) are independent predictors of access-related vascular intervention ( Table 2 ). Access-related vascular injury occurred in 33% of women compared with 18% in men and in 31% of patients without a pre-procedural CTA of the access vessels versus 19% in those with pre-procedural CTA imaging. The mean minimal diameter of the CFA was 8 ± 1.5 mm in our study population. The sheath/CFA ratio—both based on minimal diameter (OR 15.44, p = 0.004) as well as area (OR 9.90, p = 0.003) and the outer diameter of the introducer sheath ( Figure 3 )—was associated in a linear fashion with TAVR-related vascular injury ( Table 2 , Figure 3 ). The presence and volume of calcium at the puncture site were not associated with an increased incidence of vascular injury. However, TF-TAVR was performed in 4 patients with a combined sheath/CFA ratio (area) >0.90 and anterior wall calcium deposit(s), resulting in 2 access-related vascular injuries (n = 2 of 4, 50%) with the need for subsequent vascular intervention.
| Univariate analysis OR (95% CI) | P-value | Multivariable analysis OR (95% CI) | P-value | |
|---|---|---|---|---|
| Clinical Predictors (N = 333) | ||||
| Age | 1.03 (0.99-1.07) | 0.135 | – | – |
| Female | 2.22 (1.32-3.67) | 0.002 | 2.18 (1.29-3.67) | 0.004 ∗ |
| Arterial hypertension | 1.12 (0.62-2.02) | 0.712 | – | – |
| Hypercholesterolemia | 0.83 (0.49-1.38) | 0.468 | – | – |
| Diabetes mellitus | 0.90 (0.48-1.69) | 0.749 | – | – |
| Body mass index, kg/m 2 | 1.02 (0.96-1.07) | 0.539 | – | – |
| Coronary artery disease | 0.80 (0.47-1.36) | 0.410 | – | – |
| Atrial fibrillation | 1.22 (0.70-2.13) | 0.483 | – | – |
| Peripheral artery disease | 1.08 (0.51-2.25) | 0.849 | – | – |
| GFR < 60 mL/min/1.73 m2 | 1.03 (0.59-1.79) | 0.928 | – | – |
| GFR < 30 mL/min/1.73 m2 | – | – | – | – |
| Chronic obstructive lung disease | 0.72 (0.36-1.41) | 0.336 | – | – |
| NYHA class | 0.93 (0.59-1.47) | 0.761 | – | – |
| Left ventricular ejection fraction | 1.01 (0.99-1.04) | 0.238 | – | – |
| Mean gradient | 1.00 (0.99-1.02) | 0.810 | – | – |
| Aortic valve area | 1.10 (0.21-5.78) | 0.907 | – | – |
| Absence of pre-procedural access CTA | 1.96 (1.19-3.23) | 0.009 | 2.04 (1.22-3.45) | 0.007 ∗ |
| Left side access | 0.91 (0.44-1.88) | 0.795 | – | – |
| Sheath size | 1.78 (0.92-3.48) | 0.089 | 1.83 (0.90-3.75) | 0.097 |
| CTA predictors (N = 171) | ||||
| Minimal diameter | 0.68 (0.51-0.90) | 0.008 ∗ | – | – |
| Minimal area | 0.18 (0.01-0.30) | 0.005 ∗ | – | – |
| Sheath/CFA ratio (diameter) | 15.44 (2.54-98.37) | 0.004 ∗ | – | – |
| Sheath/CFA ratio (area) | 9.90 (2.24-43.78) | 0.003 ∗ | – | – |
| Visual calcium-anterior | 0.93 (0.44-1.96) | 0.855 | – | – |
| Volume calcium-anterior | 1.00 (0.99-1.02) | 0.873 | – | – |
| Visual calcium-posterior | 1.11 (0.75-1.64) | 0.597 | – | – |
| Volume calcium-posterior | 1.00 (0.99-1.00) | 0.550 | – | – |
| Visual calcium-total | 1.09 (0.71-1.68) | 0.698 | – | – |
| Volume calcium-total | 1.00 (0.99-1.00) | 0.498 | – | – |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


