Fetal and Perinatal Cardiology
Shaine A. Morris
Shiraz A. Maskatia
Carolyn A. Altman
Nancy A. Ayres
Introduction
The diagnosis of heart disease in utero has significantly evolved over the last 50 years, since the initial report of detecting a fetal heartbeat by ultrasonography in 1965 (1). As technology improved, fetal arrhythmias could be delineated (2,3,4), visualizing the anatomy of the fetal heart became feasible (5,6,7,8,9,10), and the diagnosis of fetal congenital heart disease (CHD) became possible (11,12,13,14). The previous author of this chapter, Dr. Charles S. Kleinman, was one of the pioneers of fetal cardiology. His contributions to the field were immense, starting with his first manuscript on fetal echocardiography published in 1980 (15). Dr. Kleinman and many others led the way to today’s sophisticated era, in which detailed anatomy of the heart can be visualized as early as the first trimester of pregnancy, fetal arrhythmias are routinely treated successfully, and early diagnosis of complex lesions can allow for planned delivery and potentially better outcomes for infants and families.
In this chapter we will review the implications of fetal diagnosis, fetal cardiac screening guidelines, indications for more detailed fetal echocardiography, and normal and abnormal fetal cardiovascular anatomy. Detailed discussion is included regarding the most common congenital heart lesions, fetal arrhythmias, and current research regarding fetal parental counseling.
Implications of Fetal Diagnosis of Cardiovascular Disease
In the current era, major CHD may be accurately diagnosed by fetal echocardiography in up to 95% of cases (16,17,18). However, in population studies, the most recently reported rates of prenatal diagnosis of even major lesions are much lower and highly variable by practice and region, ranging from 20% to 42% (19,20,21,22,23). The significant variation is likely due to a combination of factors including limited access to prenatal care, technical limitations, experience of the sonographer or the medical care provider, time constraints, and patient-specific factors such as poor acoustic windows.
Parental Preparation
Fetal diagnosis of heart disease allows providers to better prepare and care for the family. One of the primary benefits for the family is the ability to learn about the condition and to decide where to seek treatment in a nonurgent manner compared to when the diagnosis is made postnatally. Families may discuss whether or not to continue the pregnancy, and this decision may be informed by additional testing including detailed extracardiac imaging and genetic studies (24). Parents not only have the opportunity for extensive counseling from both perinatology and pediatric cardiology, but also have the ability to meet with other specialists including cardiovascular surgeons, neonatologists, social workers, financial counselors, lactation specialists, nurses, and psychologists or psychiatrists if appropriate (25,26,27). Nonetheless, while prenatal diagnosis has many benefits for parental preparation, some studies suggest mothers with a prenatal diagnosis may have poorer bonding with the infant, possibly secondary to subconscious attempts to protect themselves emotionally in case of infant death (28). Fetal counseling for prenatally detected CHD is more extensively discussed later in the chapter.
Morbidity and Mortality
A prenatal diagnosis allows the medical team to optimize care for the neonate based on the anticipated hemodynamic status. This includes the choice of treatment center, planned delivery close to this facility, optimal early management to prevent hemodynamic deterioration, and prompt intervention if necessary (29,30,31,32,33). Measures of morbidity including oxygenation, myocardial function, blood pH, renal function, and perioperative neurologic events have been shown to be more optimal in prenatally diagnosed infants compared to those postnatally diagnosed (34,35,36,37,38,39,40).
The question of whether prenatal diagnosis improves mortality has been elusive and controversial. Studies in which CHD lesions are grouped together suggest prenatal diagnosis is associated with increased mortality, but these findings are likely due to the lack of stratifying groups by lesion and the inclusion of higher mortality lesions in the prenatally diagnosed group (19,41). Most published studies also have the significant limitation of care at a single tertiary care facility that does not account for death prior to transfer or prior to surgery.
In hypoplastic left heart syndrome (HLHS), studies have demonstrated conflicting results regarding the potential mortality benefit from prenatal diagnosis (34,35,39). A large population-based study suggested that it is not the prenatal diagnosis itself, but delivery close to a surgical center that confers decreased preoperative mortality in HLHS (30). The authors showed more than a 6-fold greater preoperative mortality in neonates born more than 90 minutes from a cardiac surgical center, while postnatally diagnosed patients born close to a surgical center had preoperative mortality similar to those prenatally diagnosed. While several studies of transposition of the great arteries (TGA) have failed to show statistical significance in mortality among those prenatally and postnatally diagnosed, two large studies have demonstrated a significantly decreased mortality for infants who are prenatally diagnosed (32,39,42,43,44). The challenges in achieving statistical significance in studies of TGA are the low rate of prenatal diagnosis and low mortality. Although studies have suggested improved survival after prenatal diagnosis of coarctation of the aorta, a benefit has yet to be shown in right-sided lesions, complex single ventricle disease, and congenitally corrected TGA (36,38,45,46,47,48,49).
Fetal Cardiovascular Circulation
Our understanding of human fetal circulation and changes attributable to cardiovascular disease was originally derived from early work with fetal lambs (50,51,52). The introduction of fetal ultrasound and most recently fetal cardiac magnetic resonance imaging (MRI) have further refined our understanding (53,54). Unlike the postnatal heart in which blood flow is in series, prenatally, the right and left fetal ventricular outputs run in parallel (Fig. 5.1). Starting
from the placenta, maternal oxygenated blood courses through the umbilical vein and enters the inferior vena cava via the ductus venosus. This oxygenated blood accounts for approximately 50% of blood returning to the right atrium (Fig. 5.2). Oxygenated blood enters the right atrium, and then is directed across the fetal foramen ovale to the left atrium by the Eustachian valve. Most blood flow to the left side of the heart is provided in this manner (54) (∼63% per MRI estimates). Blood then courses from the left atrium across the mitral valve to the left ventricle (LV) and is ejected across the aortic valve into the ascending aorta. Blood from the ascending aorta is then directed to the brain and upper extremities (73% of aortic output) while the rest is ejected to the inferior portion of the fetal body (27% of aortic output) and joins blood from the ductus arteriosus in the descending aorta.
from the placenta, maternal oxygenated blood courses through the umbilical vein and enters the inferior vena cava via the ductus venosus. This oxygenated blood accounts for approximately 50% of blood returning to the right atrium (Fig. 5.2). Oxygenated blood enters the right atrium, and then is directed across the fetal foramen ovale to the left atrium by the Eustachian valve. Most blood flow to the left side of the heart is provided in this manner (54) (∼63% per MRI estimates). Blood then courses from the left atrium across the mitral valve to the left ventricle (LV) and is ejected across the aortic valve into the ascending aorta. Blood from the ascending aorta is then directed to the brain and upper extremities (73% of aortic output) while the rest is ejected to the inferior portion of the fetal body (27% of aortic output) and joins blood from the ductus arteriosus in the descending aorta.
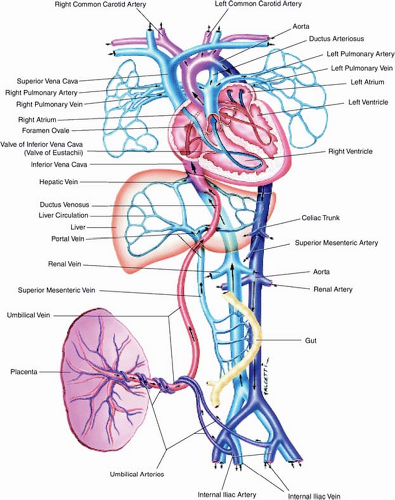 Figure 5.1 Fetal circulation. (Reprinted with permission from Uflacker R. Atlas of Vascular Anatomy an Angiographic Approach. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007.) (55). |
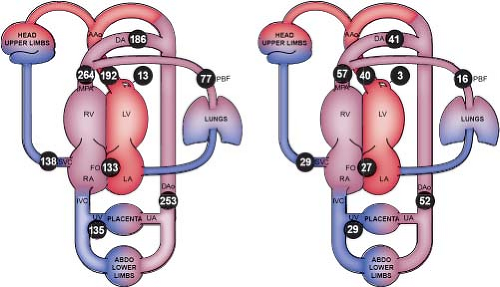 Figure 5.2 Distribution of the normal human fetal circulation measured by phase-contrast MRI in 40 late-gestation fetuses expressed as mean flows in mL/min/kg (left) and converted to modeled mean percentages of the combined ventricular output (right). Coronary blood flow estimated based on fetal lamb findings, FO flow calculated as the difference between LV output and PBF. AAo, ascending aorta; DA, ductus arteriosus; DAo, descending aorta; FO, foramen ovale; LA, left atrium; LV, left ventricle; MPA, main pulmonary artery; PBF, pulmonary blood flow; RA, right atrium; RV, right ventricle; SVC, superior vena cava; UA, umbilical artery; and UV, umbilical vein. (Courtesy of Luke Itani, The Hospital for Sick Children, Toronto, Canada. Reprinted with permission: Prsa M, Sun L, van Amerom J, et al. Reference ranges of blood flow in the major vessels of the normal human fetal circulation at term by phase-contrast magnetic resonance imaging. Circ Cardiovasc Imaging. 2014;7:663–670.) (54). |
Some of the blood that was delivered to the lower-extremity organs and muscles eventually returns deoxygenated to the heart via the inferior vena cava, while some is returned to the placenta via the umbilical artery (∼29% of combined cardiac output [CCO]). Deoxygenated blood, with a lower glucose content returning through the fetal systemic veins, courses through the right atrium, right ventricle (RV), and across the pulmonary valve (PV) into the pulmonary artery, and primarily traverses the ductus arteriosus into the descending aorta. A small proportion of blood in fetal life is directed into pulmonary circulation (∼28% of pulmonary output, 16% of CCO), and returns to the left atrium via the pulmonary veins. Three percent of cardiac output supplies the coronary arterial system. This normal fetal circulation essentially allows the most oxygen-rich, glucose-rich blood to reach the fetal brain, which may be significantly altered when CHD is present.
At the time of birth, when flow from the placenta is interrupted with cutting the umbilical cord, the neonate starts to breathe. Most of the flow from the pulmonary artery that was directed across the ductus arteriosus in fetal life is now directed into the pulmonary arteries to feed the newly expanded lungs. This blood becomes oxygenated, and returns to the left atrium via the pulmonary veins. As oxygen levels rise and prostaglandin levels fall, the ductus arteriosus closes. With increased left atrial filling from the pulmonary veins and decreased return from the ductus venosus, the flap valve of septum primum closes the foramen ovale in most infants.
Early Gestation Fetal Cardiac Evaluation and Screening for Fetal Cardiovascular Disease
CHD is the most common birth defect and the most lethal (56,57). In fetal life, the incidence of CHD is likely to be even higher due to affected fetuses undergoing an early spontaneous loss, stillbirth, or elective termination of pregnancy. Therefore, evaluation of the fetal heart must be a critical component of fetal imaging. Ultrasound evaluations that typically include evaluation of the heart are performed in the first trimester, during which cardiac activity is confirmed, as well as the standard second or third trimester study in which cardiac anatomy is evaluated from standard views (58).
The standard second or third trimester obstetric sonogram includes an evaluation of fetal presentation, amniotic fluid volume, cardiac activity, placental position, fetal biometry, and fetal number, plus an anatomic survey (58). This anatomic survey includes standardized views of the heart developed to screen for significant CHD. Earlier practice guidelines for fetal cardiac screening encouraged visualization of the four-chamber view or “basic scan” with inclusion of the cardiac outflow tracts as part of an “extended basic scan” only if technically feasible (59,60). However, multiple studies have shown that this strategy results in poor detection of potentially critical outflow tract abnormalities, such as TGA (61,62). Additional assessment of both ventricular outflow tracts can improve the detection rate of significant anomalies to approximately 80% (63,64,65). Given this, the most recent United States obstetric guidelines now include imaging of the right and ventricular outflow tracts as part of the standard examination (58). When there is either a fetal cardiac anomaly suspected, or an increased maternal or fetal risk of fetal cardiovascular disease (indications detailed below), a fetal echocardiogram is performed, typically after 18 weeks of gestation (29).
For some higher-risk families, a more detailed first trimester screen at 11 to 13 weeks of gestation may be performed with the intent of better identifying chromosomal abnormalities and birth defects (58). In these studies, nuchal translucency (NT) is measured, maternal serum tests may be drawn, and detailed cardiac evaluation may be performed. Cardiovascular assessment in early gestation (<18 weeks) may also include evaluation for extracardiac features known to have an association with cardiovascular disease, as well as two-dimensional (2D) and color Doppler imaging of the heart. Features known to be associated with increased prevalence of cardiovascular disease
include increased NT, extracardiac anomalies, and abnormal spectral Doppler waveforms in the ductus venosus (66,67,68,69,70). Other findings that have been associated with increased prevalence of fetal cardiac disease include tricuspid regurgitation, abnormal cardiac axis, and the presence of an aberrant right subclavian artery (69,71,72,73,74).
include increased NT, extracardiac anomalies, and abnormal spectral Doppler waveforms in the ductus venosus (66,67,68,69,70). Other findings that have been associated with increased prevalence of fetal cardiac disease include tricuspid regurgitation, abnormal cardiac axis, and the presence of an aberrant right subclavian artery (69,71,72,73,74).
The standard cardiac views that are typically performed during second trimester cardiac screening can also usually be performed after 11 weeks of gestation in the first trimester and early second trimester (60,75,76,77,78). The accuracy of diagnosis in early fetal echocardiography varies by gestational age; one study reported a 20% accuracy at an 11-week ultrasound compared to 92% at 13 weeks (76). In another study in high-risk women, using a combination of transabdominal and transvaginal ultrasonography, the authors reported a 70% sensitivity and 98% specificity in the identification of CHD before 16 weeks (78). However, early fetal cardiac assessment may be limited by conditions that actually progress with advancing gestational age, such as aortic stenosis (AS) and Ebstein anomaly and so it cannot be used in isolation (79).
Indications for Fetal Echocardiography
Indications are well described and divided into fetal, maternal, and familial related categories (29,80,81,82). A detailed list of indications, including estimated risk of cardiovascular disease, can be found in a multidisciplinary Scientific Statement from the American Heart Association (29). Some of the strongest or most common associations are detailed below.
Fetal Indications
Increased Nuchal Translucency
NT is a fluid-filled appearance of the back of the fetal neck measured between 11 and 13 weeks 6 days of gestation. An NT measurement of ≥3.5 mm, which is >95% of expected, is associated with increased risk of CHD, both in the absence and presence of chromosomal abnormalities, and the risk of CHD increases with increasing NT size (80,83).
Suspected CHD on Routine Obstetric Ultrasound
CHD is most frequently detected in the low-risk maternal population presenting with an abnormal obstetric screening ultrasound. The four-chamber view is excellent for detection of single ventricle lesions, hypoplastic ventricles, atrioventricular septal defects (AVSDs), and hypoplastic or atretic atrioventricular valves. Screening the outflow tracts has recently been added to the standard second or third trimester ultrasound resulting in significant increases in detection rates for TGA, truncus arteriosus, tetralogy of Fallot (TOF), double-outlet RV, and some forms of coarctation of the aorta (32,62,80,84).
Extracardiac Birth Defects
Extracardiac defects are frequently associated with CHD, and both syndromic and nonsyndromic fetuses with CHD have an increased incidence of extracardiac defects (85). Cardiac defects are observed in 30% of patients with omphalocele, 20% of those with duodenal atresia, and 30% of those with diaphragmatic hernia (86,87,88). The reported percentage of time that CHD is present with extracardiac birth defects is listed in Table 5.1.
Risk of High Output Failure
Conditions which put the fetus at risk for high output cardiac failure should trigger a fetal echocardiogram. These include monochorionic diamniotic twins with twin-to-twin transfusion syndrome (TTTS), twin reversed arterial perfusion sequence, fetal anemia, and chorioangioma.
Maternal Indications
Diabetes Mellitus
Diabetes is a common condition complicating pregnancy, affecting an estimated 3% to 10% of mothers. Maternal diabetes is associated with an approximately 5-fold increase in CHD compared to the normal population (95,96). For those with pregestational diabetes, preconception metabolic status may affect the risk of CHD. The risk of
CHD appears to increase with the hemoglobin A1C levels measured in the first trimester (95). Offsprings of diabetic mothers have an increased incidence of many types of CHD, including conotruncal anomalies including truncus arteriosus, TGA, complex single ventricles, and heterotaxy syndromes (95).
CHD appears to increase with the hemoglobin A1C levels measured in the first trimester (95). Offsprings of diabetic mothers have an increased incidence of many types of CHD, including conotruncal anomalies including truncus arteriosus, TGA, complex single ventricles, and heterotaxy syndromes (95).
TABLE 5.1 Percentage of Time Congenital Heart Disease is Present with Extracardiac Birth Defects | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Phenylketonuria
Maternal phenylketonuria (PKU) in which there is a serum phenylalanine level >15 mg/dL have a 10- to 15-fold increase risk of CHD in the fetus (97). If maternal phenylalanine levels are <6 mg/dL before conception and during the first trimester, the risks of CHD are low (98). However, if the level is >10 mg/dL a fetal echocardiogram is indicated (98,99).
Maternal Autoimmune Disease
Complete heart block (CHB) occurs in ∼4% of pregnancies when maternal autoimmune antibodies are detected in an asymptomatic or symptomatic mother. When the mother has had a prior child with CHB, the risk of CHB in the subsequent pregnancy is approximately 19% (100,101). Other cardiovascular abnormalities related to maternal autoantibodies include myocarditis, cardiomyopathy, endocardial fibroelastosis, ventricular arrhythmias, and dysplasia of the atrioventricular or semilunar valves (102).
Family History of Congenital Heart Disease
When a parent or sibling of the fetus has a history of CHD, the risk of CHD in the fetus is increased compared to the overall population, although this varies by lesion and relation. Overall, estimates of CHD when there is maternal heart disease are 5.7%, paternal heart disease 2.2%, and prior child with heart disease ∼2% to 6% (103,104).
When examining recurrence by lesion, a bicuspid aortic valve (BAV) is among the highest, with ∼9% of children having a BAV if either parent or a sibling has BAV (106). A history of AVSD in either parent is associated with a 7.7% to 7.9% birth incidence of CHD in the offspring (103). For TOF, there appears to be a higher recurrence rate if the mother is affected (∼4.5%) compared to the father (1.6%), and a similar pattern is seen in isolated pulmonary stenosis when the mother is affected (3.7%) compared to the father (1.7%) (107). Ventricular septal defects (VSDs) have a reported recurrence rate of 2% to 10%, and in some studies appear to be similar whether present in mother or father, but in others appear to occur at a higher frequency if the mother is affected (104,107).
The Fetal Echocardiogram
The goal of fetal echocardiography is to prenatally diagnose fetal cardiovascular conditions with high sensitivity and specificity to allow optimal care of the mother and fetus at delivery. Multiple practice guidelines have been published in the United States regarding fetal echocardiography, most recently by the American Institute of Ultrasound in Medicine in 2013 and the American Heart Association in 2014 (29,82).
Timing of the Fetal Echocardiogram
Timing of the fetal echocardiogram depends on the specific lesion and the facilities available in an individual institution. While 18 to 22 weeks of gestation is generally considered to be optimal, earlier evaluation may be indicated if confirmation of fetal cardiac disease may affect the family’s decision about termination (29,108). However, it is important to keep in mind that fetal echocardiography at 18 to 22 weeks may miss cases in which disease is progressive or occurs late in gestation, for example, maternal diabetes-associated ventricular hypertrophy (109). If fetal heart disease is suspected later in pregnancy, fetal echocardiography should be performed promptly to aid decision-making about delivery and potentially to guide in utero therapy. This is especially true for fetal arrhythmias, which often do not manifest before 25 to 26 weeks of gestation and, in some cases, only in the third trimester (29).
Fetal Views
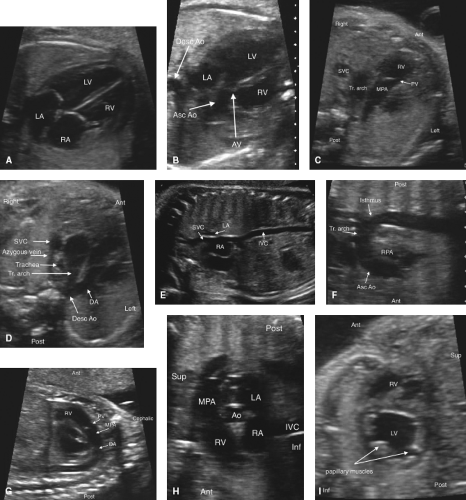
Fetal echocardiography consists of a sequential segmental analysis of the cardiovascular structures (Figs. 5.3–5.6). Evaluation in standard planes can be useful, including the (1) Four-chamber view (Videos 5.1 and 5.2), (2) left ventricular outflow tract view, (3) right ventricular outflow tract (RVOT) view, (4) three vessel and trachea view, (5) bicaval view, (6) long axis of the aortic arch, (7) long axis of the ductal arch, (8) a high short axis view of the great arteries, and (9) a low short axis view of the ventricles. Views 1 to 4 can be acquired in a long superior sweep starting in an axial plane just superior to the fetal diaphragm (Fig. 5.3; Video 5.3). In addition to gray scale imaging in all views, color Doppler ultrasonography is required with the adjunctive use of pulsed Doppler ultrasonography as needed. Measuring cardiac structures is optional although this should be considered for suspected structural or functional anomalies. Reference values have been published for most fetal cardiac structures, and gestational age-based or size-based z-score calculators are available (OBSONO.org, fetal.parameterz.com) using published reference values (110,111,112,113,114). This detailed assessment may include an advanced assessment of cardiac function such as ventricular strain and myocardial performance index (MPI) measurements.
Four-Chamber View
This is one of the most useful views in the fetal echocardiogram and is achieved by obtaining a near-transverse/axial view of the fetal heart superior to the diaphragm and inferior to the bronchial bifurcation (Figs. 5.3 and 5.6A; Videos 5.1–5.3). In addition to assessing the cardiac position and the sizes of the atria, ventricles, and atrioventricular valves, pulmonary venous return to the left atrium can be visualized. Color Doppler should be used to assess atrioventricular valvar regurgitation and to confirm pulmonary venous return.
Left-Ventricular and Right-Ventricular Outflow Tract Views
These views are performed by sweeping slightly cephalad from the four-chamber view (Figs. 5.3 and 5.6B, C; Video 5.3). The ventriculoarterial connections can be examined and narrowing of the outflow tracts, semilunar valves, and supravalvar areas can be detected by 2D imaging. Color Doppler imaging can support this finding, demonstrate valvar regurgitation, and may confirm a lack of flow in cases of semilunar valve atresia.
Three-Vessel and Tracheal View
By sweeping even further cephalad from the outflow tract views, this view can be obtained (Figs. 5.3 and 5.6D, Video 5.3). Here, the side of the superior vena cava (SVC) and the aortic arch can be defined. The transverse arch and ductal arch are visualized, and by color Doppler, flow reversal in the arch or the duct may be seen if present.
Bicaval View
The bicaval view is obtained by sagittal imaging of the fetal chest, just to the left of midline (Figs. 5.4 and 5.6E). This view will confirm that the suprahepatic portion of the inferior vena cava is intact (it may be interrupted in heterotaxy), and that a right SVC is present. This view is also the best to visualize the foramen ovale. Color Doppler should be used to assess the direction of flow across the foramen ovale,
which is normally from right to left, but may be reversed in cases of HLHS or other complex lesions.
which is normally from right to left, but may be reversed in cases of HLHS or other complex lesions.
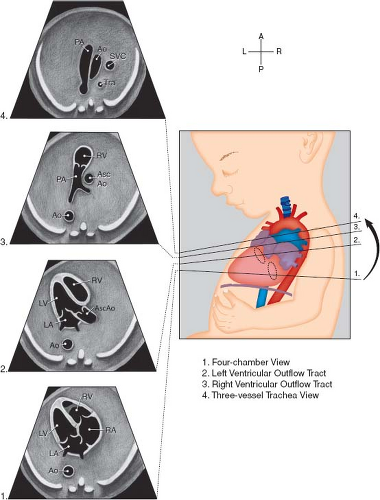 Figure 5.3 Standardized transverse scanning planes for fetal echocardiography include an evaluation of the four-chamber view (1), arterial outflow tracts (2 and 3), and the three-vessel and trachea view (4). Ao; descending aorta; Asc Ao, ascending aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle; Tra, trachea. (Reprinted with permission from American Institute of Ultrasound in Medicine. AIUM practice guideline for theperformance of fetal echocardiography. J Ultrasound Med 2013;32:1067–1082.) (81). |
Long Axis View of the Aortic Arch
The long axis view of the aortic arch, the so-called “candy-cane” view, is obtained by sagittal imaging of the fetal thorax and angling slightly leftward (Figs. 5.4 and 5.6F). It can also be obtained by aligning the transverse arch with the ultrasound beam in the three-vessel and trachea view, and turning the probe 90 degrees. The arch should be assessed for hypoplasia or coarctation, and measurements can be performed and compared with those normal for age (115). Flow should be toward the descending aorta. Reversal of flow in the arch indicates that the ductus arteriosus is supplying some of the flow to the head and upper extremity, and may be seen when there are hypoplastic left-sided
structures or an atrial septal aneurysm obstructing mitral valve inflow (116).
structures or an atrial septal aneurysm obstructing mitral valve inflow (116).
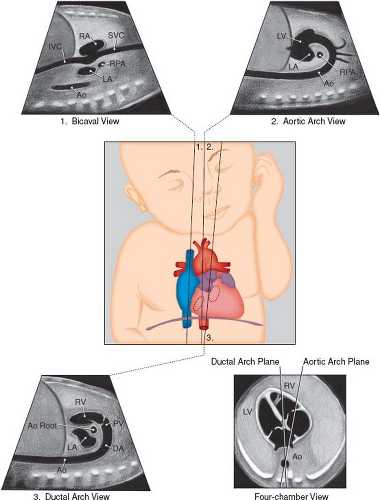 Figure 5.4 Sagittal views of the superior and inferior venae cavae (1), aortic arch (2), and ductal arch (3). The scan angle between the ductal arch and thoracic aorta ranges between 10 and 19 degrees during pregnancy, as illustrated by the four-chamber view diagram. Ao, Desc Ao, descending aorta; Ao Root, aortic root; DA, ductus arteriosus; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; PV, pulmonary valve; RA, right atrium; RPA, right pulmonary artery; RV, right ventricle; SVC, superior vena cava. (Reprinted with permission from American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of fetal echocardiography. J Ultrasound Med. 2013;32:1067–1082.) (81). |
Long Axis View of the Ductal Arch
The long axis view of the ductal arch, the so-called “hockey stick” view, is usually obtained from a direct sagittal view of the fetal thorax, just left of center (Figs. 5.4 and 5.6G; Video 5.4). The ductus should be assessed for restriction and direction of flow. By spectral Doppler, a restrictive ductus arteriosus typically has a peak velocity greater than 2 m/s and an abnormal flow pattern with continuous flow, as opposed to the normal pulsatile flow. Continuous high-velocity flow is also usually evident by color Doppler imaging. Flow reversal in the ductus arteriosus is present with severe right-sided obstructive lesions, such as severe pulmonary stenosis or pulmonary atresia (PA).
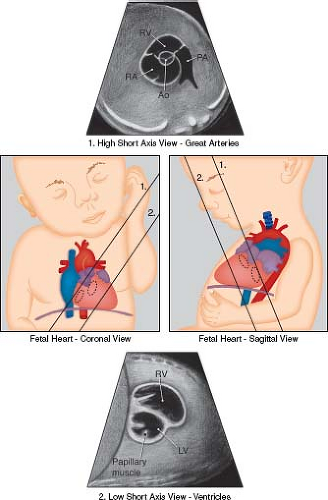 Figure 5.5 Low and high short-axis views of the fetal heart. Ao, aortic valve; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle. (Reprinted with permission from American Institute of Ultrasound in Medicine. AIUM practice guideline for theperformance of fetal echocardiography. J Ultrasound Med. 2013;32:1067–1082.) (81). |
High Short Axis View of the Great Arteries
Imaging in cross-section of the heart can be very useful in assessing the outflow tracts, the ventricular septum, cardiac function, and the anatomy of the atrioventricular valves (Figs. 5.5 and 5.6H). The first short-axis view is the high short axis, which demonstrates the aortic valve en face, surrounded by the pulmonary artery wrapping around it. The pulmonary arteries can also be seen bifurcating, and the tricuspid valve is usually well seen. The high short axis view is best for demonstrating obstruction to the RVOT and for differentiating types of VSDs that involve the outlet portion of the ventricular septum. Tricuspid valve regurgitation can also be assessed from this view.
Low Short Axis of the Ventricles
The low short axis is the best view to assess ventricular function, and to evaluate for muscular VSDs (Figs. 5.5 and 5.6I). As muscular VSDs are often very small, the use of color Doppler to show flow across the septum is usually necessary to detect or confirm these defects. The morphology of the atrioventricular valves can be assessed and a common atrioventricular valve or mitral valve cleft can be detected.
Assessment of Cardiovascular Performance
One goal of fetal echocardiography is to assess cardiovascular performance. Even in the structurally normal fetal heart, there is a variety of cardiac and noncardiac pathologies that may affect the ability of the heart to deliver oxygen to the brain and other organ systems. These include perinatal infection, maternal diabetes, exposure to toxins, anemia, autoimmune disease, a mass effect due to congenital anomalies, arrhythmias, inherited or idiopathic cardiomyopathies, and increased fetal preload or afterload due to vascular anomalies, placental abnormalities, vascular tumors, or humoral factors (117,118,119,120). A precise history and assessment of noncardiac pathology present in the fetus is crucial to determine the etiology of fetal cardiovascular dysfunction.
In the fetus, as in the child and adult, cardiac output is a function of stroke volume and heart rate. Stroke volume, in turn, is determined by the preload, afterload, and contractility of the ventricles. This, however, is where the similarities end. The fetal circulation operates in parallel compared to the postnatal circulation, which operates in series. The fetal LV, instead of supporting the entire systemic circulation, is responsible only for the head and upper body. The fetal RV, on the other hand, is responsible for supporting the lower body and placental circulation (Fig. 5.1). Because of this difference, the fetal RV typically has a slightly larger cross-sectional dimension as well as a larger estimated output compared to the left (54,114,121,122,123,124). The determinants of cardiac output also appear to vary dramatically between fetal and postnatal circulations. The fetal myocardium has a markedly reduced compliance compared to the postnatal, and thus responds poorly to volume loading (125,126,127). Fetal cardiac output is also highly sensitive to afterload. In cases of placental insufficiency, increases in the afterload to the fetal RV may result in a significant reduction in output of that ventricle. The sole source of metabolic energy of the fetal myocardium is glucose, and the fetal sarcoplasmic reticulum accumulates calcium at a slower rate compared to adult myocardium (128). These findings may explain the dramatic decompensation seen in fetuses with uncontrolled tachycardia.
In the fetus, congestive heart failure can be defined as an inadequate oxygen delivery for normal end-organ function. higher extracellular water content, diminished calcium stores in the sarcoplasmic reticulum, lower albumin concentration, higher heart rate, and lower systemic arterial pressure result in a propensity for elevated ventricular filling pressures with alterations in fetal hemodynamics. Therefore, regardless of the etiology, indices of diastolic dysfunction tend to precede those of systolic dysfunction. Markers of diastolic dysfunction in the fetus include atrioventricular valvar regurgitation, umbilical venous pulsations, flow reversal in the ductus venosus, or monophasic atrioventricular valve inflow. As heart failure progresses in the fetus, fluid may accumulate in the pericardial space, in the pleural space, in the peritoneal cavity, and in the soft tissues. Fluid accumulation in two or more compartments clinically defines hydrops fetalis, a risk factor for morbidity and mortality in a variety of disease states (119,129,130). Finally, systolic dysfunction is a late and ominous finding in fetal cardiac dysfunction. While measurement of ejection fraction of the LV is possible using the area-length method, fetal systolic function is currently most often assessed by simple single plane assessment of shortening fraction. A shortening fraction of <28% is considered to be abnormal, regardless of gestational age.
The Cardiovascular Profile Score (CPS), initially proposed by Huhta, is a scoring system that incorporates cardiac and extra cardiac aspects of fetal cardiovascular function (131). The CPS encompasses five categories (hydrops, umbilical venous Doppler, heart size, abnormal myocardial function, and arterial Doppler), each
worth 2 points (Fig. 5.7). The CPS has been validated as a predictor of adverse fetal outcome in a variety of disease processes, including CHD, high-output extra cardiac lesions, TTTS, idiopathic hydrops fetalis, and primary fetal cardiomyopathy (132,133,134,135,136,137,138,139). The cutoff point for predicting poor fetal outcome, however, has varied between studies, and has ranged from <6 to <10.
worth 2 points (Fig. 5.7). The CPS has been validated as a predictor of adverse fetal outcome in a variety of disease processes, including CHD, high-output extra cardiac lesions, TTTS, idiopathic hydrops fetalis, and primary fetal cardiomyopathy (132,133,134,135,136,137,138,139). The cutoff point for predicting poor fetal outcome, however, has varied between studies, and has ranged from <6 to <10.
The MPI, or Tei Index, is a commonly used measure of cardiac function on the fetal echocardiogram. The MPI is obtained by measuring the AV valve closure time to opening time (systole), and the semilunar valve ejection time (Fig. 5.8). The ejection time is then subtracted from the AV valve closure to opening time in order to arrive at the sum of isovolumetric contraction and relaxation. This sum is then divided by the ejection time. Normal values of the MPI change slightly throughout gestation. Generally, values of less than 0.43 to 0.45 are considered to be normal (140). Elevated MPI values can reflect an alteration in both systolic and diastolic function.
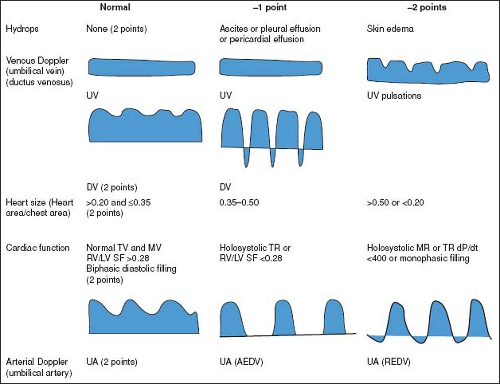 Figure 5.7 Components of the Cardiovascular Profile Score. The heart failure score is 10 if there are no abnormal signs and reflects 2 points for each of five categories: hydrops, venous Doppler, heart size, cardiac function, and arterial Doppler. AEDV, absent end-diastolic velocity; dp/dt, change in pressure over time of TR jet; DV, ductus venosus; LV, left ventricle; MR, mitral valve regurgitation; MV, mitral valve; SF, ventricular shortening fraction; TR, tricuspid valve regurgitation; TV, tricuspid valve; REDV, reversed end-diastolic velocity; RV, right ventricle; UV, umbilical vein. (Reproduced with permission from Falkensammer CB, Paul J, Huhta JC. Fetal congestive heart failure: correlation of Tei-Index and Cardiovascular-Score. J Perinat Med. 2001;29(5):390–398.) (124). |
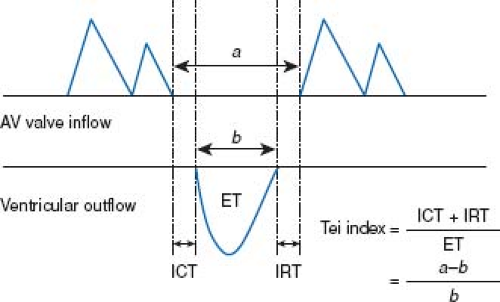 Figure 5.8 Measurements of Doppler time intervals. The Tei index is calculated as (a−b)/b. The time interval a is from cessation to onset of atrioventricular (AV) valve inflow, which is equal to the sum of isovolumetric contraction time (ICT), ejection time (ET), and isovolumetric relaxation time (IRT). Ventricular ET (b) was measured as the duration of left or right ventricular outflow. (Reproduced with permission from Sugiura T, Suzuki S, Hussein MH, Kato T, Togari H. Usefulness of a new Doppler index for assessing both ventricular functions and pulmonary circulation in newborn piglet with hypoxic pulmonary hypertension. Pediatr Res. 2003;53(6):927–932.) (141). |
Cardiac output can be estimated by echocardiography from the product of the cross-sectional area of the semilunar valve, the heart rate, and the velocity time integral. In the fetus, due to the parallel circulations, the aortic cardiac output must be added to the pulmonary to arrive at an estimated CCO (121,142). Fetal estimates of CCO have correlated with CPS in high cardiac output lesions such as sacrococcygeal teratoma (134) and with worse outcomes in TTTS (143).
Congenital Heart Lesions
Ventricular Septal Defects
Medium to large VSDs can be detected in the fetus with careful imaging. However, VSDs are among the most commonly missed lesions by prenatal imaging, presumably due to the wide variation in size, and equal ventricular pressures precluding easy observation of shunting (144). Smaller defects may be visualized, but it can be much more challenging to distinguish these from artifact. In both early and later gestation, a combination of 2D ultrasonography and Doppler color imaging can help to identify VSDs and to minimize false positives (145). Although large defects can often easily be visualized by 2D imaging, the diagnosis should always be confirmed by color Doppler imaging, which usually demonstrates bidirectional flow across the defects.
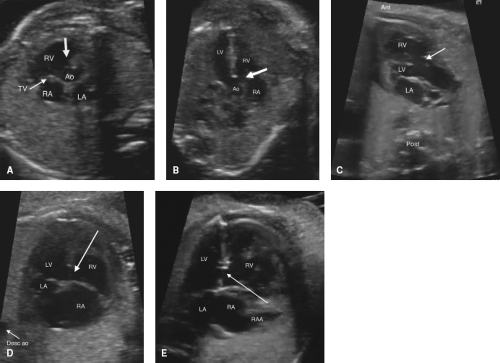
Perimembranous VSDs
Perimembranous VSDs are seen from several views, including the left ventricular outflow tract view and high short axis views (Fig. 5.9A–C). False positives are high, as the membranous septum is the thinnest part of the septum, and can appear to be missing even when present. A perimembranous defect should always be confirmed by color Doppler. Perimembranous defects may be missed if redundant tricuspid valve tissue covers the defect, preventing visualization of shunting. With normal segmental anatomy, the aortic valve and/or tricuspid valve will always be in the image as a border of the defect. If one starts from a four-chamber view, and sweeps cephalad, the defect is visible just as aortic valve comes into view.
Muscular outlet extension of a perimembranous VSD associated with malalignment of the infundibular septum is frequently seen as part of TOF (anterior malalignment, Fig. 5.9C) or interrupted aortic arch (posterior malalignment). There is also a higher association with a right aortic arch which can be detected and may trigger further genetic workup.
Isolated defects in the inlet septum that border the atrioventricular valves are termed perimembranous inlet type by Anderson (atrioventricular canal type VSDs by Van Praagh). This type of defect is best diagnosed in the four-chamber view (Fig. 5.9D). One should carefully inspect for a primum ASD, common atrioventricular valve, or zone of apposition in the left atrioventricular valve to ensure that the defect is not a component of an AVSD.
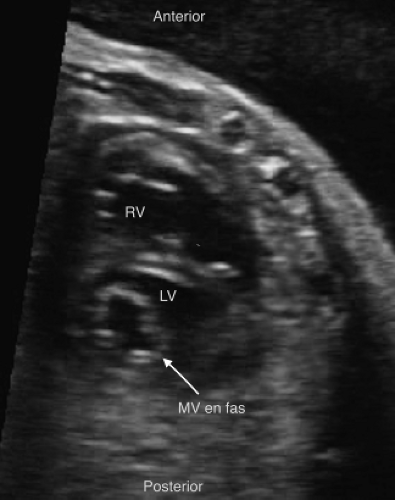
Muscular VSDs
Muscular VSDs are the most difficult to detect on fetal imaging, as they are often small and may have irregular borders. They are typically detected by color sweeps using a low Nyquist limit in the four-chamber and low short axis views, but may also be noted by 2D imaging without color (Fig. 5.9E; Video 5.5).
Doubly Committed Subarterial VSDs
Doubly committed subarterial VSDs are the least common type of VSD and may be confused with a membranous defect during fetal evaluation, as they are most easily seen sweeping from the four-chamber view superiorly/anterior to the outflow tracts. Some keys on fetal imaging are to remember that although one border is the aortic valve (like a perimembranous VSD), this VSD is not bordered by the tricuspid valve, but is typically bordered by the pulmonary valve, so that the aortic and pulmonary valves share a hingepoint. High short axis imaging is the best view to distinguish doubly committed subarterial from perimembranous defects.
Both muscular and perimembranous VSDs may close spontaneously, and this may occur in utero (145,146,147,148). Studies suggest that between 2% and 31% of muscular defects close during fetal life, with another 19% to 75% closing during the next 12 months (145,147,148,149). Up to 15% of muscular defects discovered in utero will require surgery (147,148). For perimembranous defects, studies suggest 4% to 35% of these close in fetal life, and another 1% to 23% in first 12 months, with an estimated 42% needing surgery (146,148,149). Malalignment VSDs and doubly committed subarterial defects rarely close spontaneously, and most often require surgical repair.
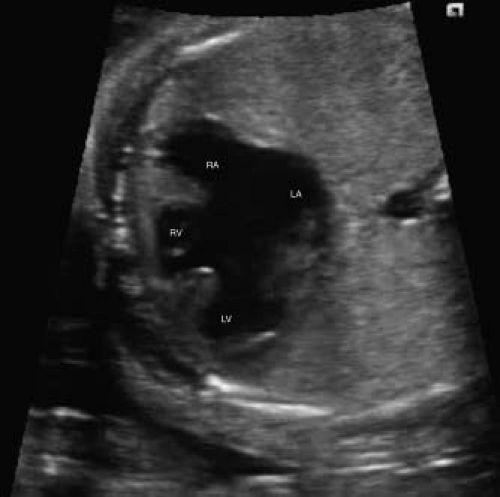
Atrioventricular Septal Defects
Atrioventricular septal defects (AVSDs) are most easily demonstrated in the four-chamber view (Fig. 5.10; Video 5.6). This allows easy visibility of the atrioventricular valve attachments, primum
atrial septal defects, as well as any ventricular septal component. Short-axis imaging across the atrioventricular valves also allows for close inspection of the left atrioventricular valve for evidence of a common valve or zone of apposition/cleft (Fig. 5.11). Color Doppler should always be used, as this may allow better detection of the degree of ventricular level shunting, as this will significantly affect counseling. Color Doppler can also be used to assess the degree of fetal atrioventricular valvar regurgitation, which has been shown to correlate closely with the degree of postnatal regurgitation (150).
atrial septal defects, as well as any ventricular septal component. Short-axis imaging across the atrioventricular valves also allows for close inspection of the left atrioventricular valve for evidence of a common valve or zone of apposition/cleft (Fig. 5.11). Color Doppler should always be used, as this may allow better detection of the degree of ventricular level shunting, as this will significantly affect counseling. Color Doppler can also be used to assess the degree of fetal atrioventricular valvar regurgitation, which has been shown to correlate closely with the degree of postnatal regurgitation (150).
When AVSD is diagnosed in utero, there must be a high suspicion of genetic abnormalities. In one study, the frequency of genetic disorders in nonheterotaxy AVSD was 74% (151). The most common diagnosis was Trisomy 21 (34 of 53 tested fetuses with AVSD), but Trisomy 18 and Turner syndrome were also associated. One study has reported spontaneous in utero closure of the VSD component in four fetuses with complete AVSD (152).
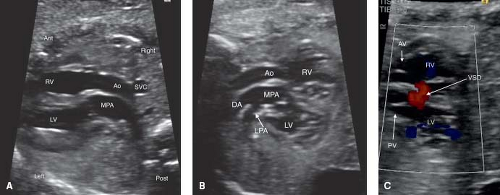
Transposition of the Great Arteries
Fetal TGA is a malformation often undetected due to a normal appearing fetal four-chamber view. Of the major congenital heart lesions, it is the least commonly detected in utero (153). Recently, the addition of outflow tract evaluation to routine screening has improved its detection (Fig. 5.12A–C; Videos 5.7, 5.8) (32).
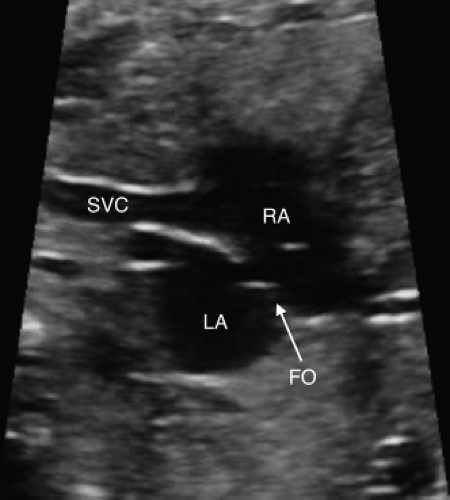
The patent foramen ovale (PFO, Fig. 5.13) and the patent ductus arteriosus (PDA) are two very important structures to evaluate in the fetus with TGA and a functionally intact ventricular septum. Abnormal flow in either a PDA and/or an abnormal or a restrictive PFO may be predictive of severe neonatal hypoxemia associated with an increased morbidity and mortality. In the fetus with TGA the highly oxygenated blood from the placenta is directed into the left heart crossing through lungs and PDA, which results in pulmonary vasodilation, deceased pulmonary vascular resistance, increased pulmonary blood flow, and increased left atrial filling pressure that can shift the atrial septum to the right or restrict the PFO. In late gestation, abnormal constriction of the PDA may occur due to the higher oxygen content of the blood traversing it. A restrictive PDA is associated with an anatomic constriction or narrowing and high velocity, turbulent, continuous antegrade flow through it. Retrograde holodiastolic laminar flow into the PDA is abnormal and also associated with neonatal hypoxemia (154,155).
In the fetus with TGA, several features of the atrial septum are considered to be predictive of neonatal hypoxemia because of inadequate mixing of blood at the atrial level, necessitating urgent newborn balloon atrial septostomy. These concerning findings include a restrictive PFO that is flat or protruding toward the right atrium, the PFO bowing into the LA greater than 50% of its width, or a hypermobile PFO undulating between the left and right atrium. Appropriate parental counseling and multidisciplinary delivery planning is required when a fetus is diagnosed with TGA and may require an urgent neonatal intervention (154,155,156,157,158,159).
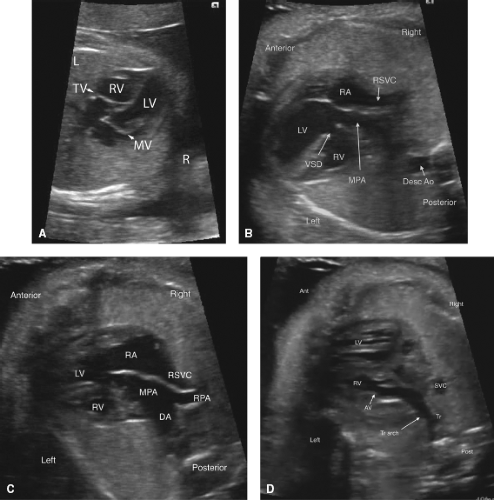
Congenitally Corrected Transposition of the Great Arteries
Congenitally corrected transposition of the great arteries (CCTGA) is frequently not diagnosed prenatally, possibly because of the relatively normal chamber sizes on the four-chamber view (Fig. 5.14A) (47). CCTGA is most easily diagnosed in the fetus utilizing sweeps in multiple directions to confirm the discordant atrioventricular and ventriculoarterial relationships (Video 5.9). In the fetus with CCTGA, coexisting lesions are very common, and include VSD (62% to 79%), pulmonary outflow tract obstruction (35% to 50%), and tricuspid (systemic AV valve) valve abnormalities, for example, Ebstein anomaly and dysplasia (25% to 36%). Thus careful inspection of the ventricular septum, atrioventricular valves, and outflow tracts is important (Fig. 5.14 B and C) (47,160,161). CHB may be present in 6% to 10% of fetuses with CCTGA; thus the
fetal heart rhythm should be evaluated in detail (47,160,161,162). CCTGA in the fetus is also associated with cardiac malposition, heterotaxy, ventricular hypoplasia (Fig. 5.14B–D), or valvar atresia (47,161,163). Only 1% to 2% of CCTGA patients have no additional cardiac anomaly (164). In two fetal series of CCTGA, spontaneous intrauterine fetal demise (IUFD) was rare, occurring on 0/14 and 1/30 fetuses; however in one study of 34 fetuses, 3 had IUFD (9%), including one with CHB (47,160,161).
fetal heart rhythm should be evaluated in detail (47,160,161,162). CCTGA in the fetus is also associated with cardiac malposition, heterotaxy, ventricular hypoplasia (Fig. 5.14B–D), or valvar atresia (47,161,163). Only 1% to 2% of CCTGA patients have no additional cardiac anomaly (164). In two fetal series of CCTGA, spontaneous intrauterine fetal demise (IUFD) was rare, occurring on 0/14 and 1/30 fetuses; however in one study of 34 fetuses, 3 had IUFD (9%), including one with CHB (47,160,161).
Tetralogy of Fallot and Pulmonary Atresia with VSD
Conotruncal defects, reviewed in depth separately, can represent a challenge to prenatal diagnosis. TOF, double outlet right ventricle (DORV), PA with a VSD, and interrupted aortic arch are all thought to result from a disturbance in the development of the conotruncus. These defects may involve abnormal septation of the aorta and pulmonary outflow tracts, abnormal alignment between the outflow tracts and the ventricles, and the presence of a VSD. The link between these defects is further highlighted by the common association with 22q11.2 deletion syndrome, many with the DiGeorge phenotype (Table 5.2). In the long-axis view of the LV, the appearance of a VSD and overriding of a great vessel can signal the presence of any of the above lesions. In addition, even in a fetus with an isolated VSD and no conotruncal abnormalities, the aorta may appear to be overriding in certain views. Distinguishing these lesions and searching for known associated lesions is of the utmost importance.
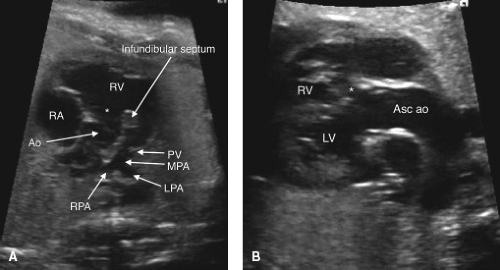
TOF is the most common cyanotic cardiac defect, with a birth prevalence of approximately 0.5 per 1000 live births (176,177). Anterior and superior deviation of the infundibular septum is a pathognomonic feature (Fig. 5.15A) (178). An aorta that overrides the ventricular septum is present (Fig. 5.15B), although this same characteristic may be present in truncus arteriosus, PA with VSD, or DORV with subaortic VSD. The RVOT view, with attention paid to the relationship between the infundibular and trabecular septum, is crucial to making the diagnosis. Color and spectral Doppler interrogation of the PV (Videos 5.10, 5.11) and of the ductus arteriosus, as well as measurement of the PV and main pulmonary artery can aid in predicting the postnatal outcome, as retrograde flow in the ductus is associated with a need for postnatal patency of the ductus arteriosus to maintain adequate pulmonary flow (179,180,181). The presence of associated cardiac anomalies, such as additional VSDs, a right-sided aortic arch, or pulmonary artery hypoplasia should be excluded.
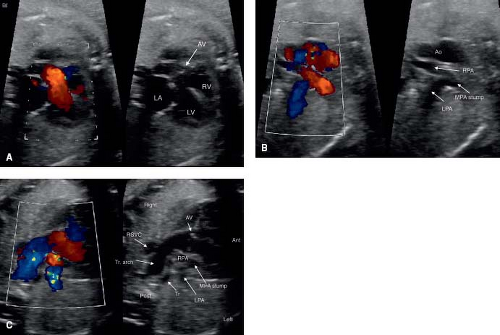
PA with a ventricular septal defect (PA-VSD) is viewed by many to be the most severe form of TOF, with complete obliteration of the RVOT. From the outflow tract views, an overriding aorta is visualized, and it can be indistinguishable from TOF (Image 16A). Determining whether the pulmonary arteries are confluent (image 16B), and the source of pulmonary flow are crucial. In the case of pulmonary
flow fed by a ductus arteriosus, prostaglandin initiation will be necessary at birth, as will neonatal surgery to establish a stable source of pulmonary blood flow (Video 5.12). Where pulmonary flow is provided by aortopulmonary collateral arteries, flow is typically stable at birth. In the case of pulmonary flow supplied by a ductus arteriosus, the ductus is often small and tortuous. A right-sided aortic arch is common in PA-VSD, and can be detected prenatally (Fig. 5.16C, Video 5.12) (165). Just as TOF may be associated with an AVSD, especially in Trisomy 21, PA-VSD may be associated with an AVSD (Video 5.13). In cases of PA-VSD with an AVSD, careful attention must be paid to systemic and pulmonary venous return, as this combination is common in heterotaxy (182).
flow fed by a ductus arteriosus, prostaglandin initiation will be necessary at birth, as will neonatal surgery to establish a stable source of pulmonary blood flow (Video 5.12). Where pulmonary flow is provided by aortopulmonary collateral arteries, flow is typically stable at birth. In the case of pulmonary flow supplied by a ductus arteriosus, the ductus is often small and tortuous. A right-sided aortic arch is common in PA-VSD, and can be detected prenatally (Fig. 5.16C, Video 5.12) (165). Just as TOF may be associated with an AVSD, especially in Trisomy 21, PA-VSD may be associated with an AVSD (Video 5.13). In cases of PA-VSD with an AVSD, careful attention must be paid to systemic and pulmonary venous return, as this combination is common in heterotaxy (182).
TABLE 5.2 Likelihood of Chromosome 22q11.2 Deletion Syndrome with Different Lesions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Truncus Arteriosus
Truncus arteriosus (also known as common arterial trunk) is a conotruncal defect characterized by a single outlet from the heart which gives risk to both the systemic and pulmonary blood flow (Fig. 5.17A and B). Truncus arteriosus can be mistaken for PA with a VSD given a similar LVOT view (Fig. 5.17C and D). Determining the source of pulmonary blood flow is essential to distinguishing these two lesions. Additionally, the identification of a dysplastic, regurgitant, truncal valve can aid in making this distinction. The ductus arteriosus is absent in the majority of cases (183). When the ductus arteriosus is present, arch interruption (Video 5.14) and ductal origin of a pulmonary artery should be excluded. Identifying aortic arch interruption is crucial, as this would signal the need for prostaglandin administration upon delivery and neonatal surgery to establish stable systemic blood flow. Follow up prenatal echocardiography is important, as progressive truncal regurgitation, fetal hydrops and in utero death may develop (184,185).
Double Outlet Right Ventricle
DORV occurs when both of the great arteries arise from the RV (186). The ventriculoarterial alignment results in both the great arteries positioned at least 50% over the RV. There is usually a loss of fibrous continuity between the mitral valve and the posterior semilunar valve. DORV is associated with a broad spectrum of intracardiac defects and great artery relationships, all of which impact on the clinical presentation and surgical management. In DORV, the outlet from the LV is via the VSD. DORV types are classified based upon VSD location. In subaortic VSD with normally related great arteries, LV output is directed to the aorta and often there is associated subpulmonary obstruction. The physiology is either that of pulmonary over circulation due to the VSD or depending on the degree of pulmonary outflow tract and pulmonary arterial obstruction, physiology akin to TOF. When the VSD is subpulmonary, the LV output is directed to the PA and the physiology is usually similar to TGA with a VSD. A subpulmonary VSD may be associated with a subaortic obstruction, coarctation of the aorta, aortic valvar stenosis or arch hypoplasia. Typically when the defect is subpulmonary the great arteries are side-by-side (Fig. 5.18, Video 5.15). DORV with an uncommitted or doubly committed VSD are the least common types. A perimembranous inlet VSD (atrioventricular canal type VSD in Van Praagh nomenclature), which is typically uncommitted is commonly associated with heterotaxy.
DORV can be undetected in the fetus when the four-chamber view appears normal (63,187). Recent inclusion of outflow track visualization to routine screening has significant potential to improve detection rates (62,80). The location of the VSD and the relationships of the great arteries can usually be accurately determined on fetal echocardiography. Genetic abnormalities are found in 14% to 20% of fetuses with DORV, although 22q11 deletion syndrome is rare (188,189). Fetuses with DORV and multiple extracardiac anomalies have the worst outcomes (190). Given this, prenatal counseling for DORV is highly variable and is based on the intracardiac anomalies, the great artery relationships and the presence of extracardiac or chromosomal abnormalities (191).
Aortopulmonary Window
Aortopulmonary window is a rare condition in which there is a communication between the ascending aorta and pulmonary trunk proximal to its bifurcation (Fig. 5.19). Common associations include interrupted aortic arch, arch hypoplasia, and VSD. Prenatal diagnosis of aortopulmonary window is uncommon, but has been reported (192,193). Two-dimensional sweeps and use of color Doppler allow visualization of an aortopulmonary window in utero (Videos 5.16, 5.17).
Pulmonary Atresia with Intact Ventricular Septum
Pulmonary atresia with an intact ventricular septum (PA-IVS) is a morphologically heterogeneous lesion characterized in which there is right ventricular outflow tract obstruction (RVOTO) either due to an atretic PV or muscular infundibular atresia (Fig. 5.20A). It is often accompanied by hypoplasia of the right ventricular cavity, due in part to increased muscularization of the RV (Fig. 5.20B). Fetal critical PV stenosis may evolve into atresia in utero (194). RV size and growth potential is variable and is related to the size of the tricuspid (TV) annulus (195). Fetal PA-IVS with TV/RV hypoplasia exhibits impaired growth as the pregnancy progresses (195,196). At mid-gestation the RV may be tripartite with a well-developed infundibulum. Where the RV is tripartite, the TV z score >−3 and the RVOT is patent, a biventricular repair is anticipated (195). A bipartite RV is a hypoplastic, hypertrophied RV which is nonapex forming. Fetus with PA-IVS and TV z score ≤−3 are more likely to undergo univentricular repair. This group with TV annular hypoplasia also have a high prevalence of RV to coronary artery fistulae (Fig. 5.20C) (196). Fetuses with coronary artery fistulae have a worse long-term prognosis due to possible RV-dependent coronary flow (195,196). In another study, a fetal score using four measurements in PA-IVS were predictive for a univentricular repair: TV/MV annulus ratio ≤0.83; PV/AV annulus ratio ≤0.75 RV/LV length ≤0.64, and TV inflow duration/cardiac cycle length ≤36.5% (197). A univentricular repair was performed in all fetuses in whom four criteria were present and in 92% of those with three criteria. Another study found that while TV annular size was a good predictor of outcome at all gestational ages, the best echocardiographic predictors varied by gestational age (198).
Fetal counseling is based upon RV and TV size and the absence or presence of coronary artery fistulae. When fistulae are present, prenatal counseling should include increased risk of fetal death, coronary artery insufficiency after delivery, and possible need for cardiac transplantation. Fetal pulmonary valvuloplasty has been shown to result in improved growth of the RV in selected fetuses with PA-IVS, and is discussed further in Section XIII (199,200,201,202).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree