Cardiovascular Intensive Care
Dean B. Andropoulos
Lara S. Shekerdemian
Paul A. Checchia
Anthony C. Chang
Introduction
Pediatric Cardiovascular Intensive Care has become increasingly organized as a subspecialty over the past two decades in response to the explosion of knowledge and research in the patient with critical cardiac disease, the increasing complexity of cardiac lesions and procedures to treat them, and the growing numbers of patients of a younger age requiring cardiac intensive care. Indeed an international subspecialty society, the Pediatric Cardiac Intensive Care Society (PCICS), was organized in 2003 to address the issues facing practitioners (1). The consensus is that critically ill pediatric patients with acquired or congenital heart disease (CHD) are best cared for in units or systems organized and designed for these patients, and by practitioners with specialized training, expertise, and skills in this area have dramatically changed the patient care paradigm. The pediatric cardiac intensivist plays a central role in the critical care of these patients. This chapter will review the organization of Pediatric Cardiac Intensive Care Unit (PCICU) services, as well as continuous quality improvement and family-centered care. Then, evaluation of cardiac output (CO) and treatment of low cardiac output syndrome (LCOS) will be addressed. Next, major organ systems as they relate to cardiac intensive care will be reviewed. Perioperative care for specific lesions and procedures will next be presented. Finally, medical conditions in the PCICU and special considerations for the neonate and adult will be reviewed.
Organization of Cardiac Intensive Care Services
Despite remarkable progress in the organization of PCICU services, there has been relatively little published information about the optimal delivery of care to this specialized patient population (2,3,4). Practitioners of the subspecialty may be pediatric cardiologists, pediatric intensivists, pediatric anesthesiologists, or pediatric cardiac surgeons. In a recent survey, 73% of North American, and 59% of worldwide PCICUs involved care by pediatric intensivists—that is, physicians with primary training in pediatric critical care (5). There are currently several paths to being a pediatric cardiac intensive care specialist. Specialized training in pediatric cardiac intensive care varies, from a fourth year of training in cardiac intensive care following a standard pediatric cardiology or intensive care fellowship in the United States, to dual fellowships in both fields, or additional dedicated months of training during a pediatric intensive care, pediatric cardiology, or pediatric cardiovascular anesthesiology fellowship. The fellows staffing a PCICU include those training in pediatric cardiology, intensive care, anesthesia, and congenital heart surgery. As of this writing there is no formal specialized certificate or board certification in PCICU in the United States. Clearly the physicians caring for PCICU patients optimally must have special interest, experience, and focus on this patient population and spend a significant amount of their clinical time caring for them in order to maintain skills, knowledge, and expertise in this complex and rapidly changing field. There is opportunity for formal curriculum development in this subspecialty (6,7). The projected significant need for PCICU physicians, and the relatively few physicians being trained, means that continued multidisciplinary support of PCICU programs is essential (2,8).
Similarly, the physical organization of PCICU beds varies considerably. In a recent survey of PCICUs in the United States and worldwide, 27 of 29 high volume centers, defined as >300 open surgical cases per year, cared for patients in dedicated PCICUs. Smaller programs were more likely to care for pediatric cardiac intensive care patients in mixed pediatric intensive care units (PICUs) or adult cardiosurgical ICUs (5). Designs range from separate, dedicated PCICUs remote from the PICU, to the PCICU having separate beds adjacent or in close proximity to the PCICU, to a mixed PICU/PCICU with cardiac patients dispersed throughout the unit. Other important design aspects considered optimal include close proximity to cardiac operating rooms and catheterization laboratories to account for the frequent patient transfers back and forth for care, and the need for rapid response and frequent communication by the practitioners in these disciplines. Proximity to imaging modalities such as computed tomography and magnetic resonance imaging is highly desirable. Bed space considerations are very important, as PCICU patients often require space for echocardiography, nitric oxide delivery systems, extracorporeal support, and surgical procedures at the bedside. Ample space for the operating room team, surgical lights, and provision for a sterile surgical field is highly desirable. In addition, space for the family is increasingly planned in modern PCICU designs. A minimum standard for bed space per patient in PICUs is 250 square feet (23 m2), but in modern PCICU designs up to 500 square feet are often planned (9). Bed spaces may be arranged as single, individual beds, to shared spaces divided by movable sliding glass partition doors, to an open-bay design for multiple patients. Careful consideration for visibility of beds and monitors should be made during design of the PCICU. Many PCICUs are designed with rectangular or square pods of 8 to 10 beds for maximum visibility and access. Adherence to local regulatory standards for windows, clean and dirty utility areas, isolation rooms, negative pressure rooms, and nutrition preparation areas are also very important. Newer design and patient flow concept have emerged in recent years, including that of a Cardiac Unit concept where patients are admitted after cardiac surgery to a private ICU room, which will be that patient’s room for the duration of the hospitalization. This concept means that acute ICU, step-down ICU, and ward levels of care are performed in the same location. The logistics of medical and nursing teams caring for patients of differing acuity levels in the same unit are complex, but this paradigm may have several advantages for family-centered care (see section “Family Centered Care”).
Although PCICUs may vary in terms of the types of patients admitted, it is important for each unit to have a specified set of admission criteria. Some units may be limited to postsurgical cardiac patients, but increasingly, PCICUs admit both medical and surgical patients with critical cardiac disease that requires constant monitoring and access to medical, nursing, and ancillary expertise. This often includes the preoperative neonate, patients with severe medical cardiac disease, and even adults with CHD after cardiac surgery.
Multidisciplinary Team Approach to Pediatric Cardiac Intensive Care
One of the most important paradigm shifts in PCICU care is the recognition that multidisciplinary care, beyond the traditional group of cardiologists, surgeons, intensivists, and anesthesiologists, is increasingly important. Additional medical disciplines of adult CHD, neonatology, neurology, gastroenterology and nutrition, general surgery, pulmonology, genetics, nephrology, and others, are vitally important to include in consultations and multidisciplinary rounds. Nursing care deserves special emphasis, as the specialized skills and knowledge in PCICU care are critically important to establish and maintain. Nursing staffing ratios must be carefully planned, and nurse to patient ratios can vary from 1:3 for less ill, nonventilated patients, to 1:1 for less stable ventilated patients, to 2:1 for ECMO patients (5). In addition, as trainee duty hours and rotations are restricted by the U.S. Accreditation Council for Graduate Medical Education and other organizations, midlevel practitioners are increasingly employed in PCICUs, including pediatric nurse practitioners and physician’s assistants. In some PCICU settings, pediatric hospitalists serve to augment manpower. Other crucial members of the multidisciplinary team (ideally dedicated and with special expertise in PCICU), are respiratory therapists, extracorporeal life support (ECLS) specialists, pharmacists, nutritionists, occupational and physical therapists, social workers, translators, and unit secretaries dedicated to the PCICU (3). Input is sought from all of these stakeholders in the patient’s care by the attending PCICU physician, and all members of the team must be given the opportunity to contribute on rounds and other formats for multidisciplinary collaboration. Multidisciplinary team planning for crises, including CPR and ECMO, which includes simulation scenarios, are important to prepare for the actual events. The parent, patient, and family are also important members of the team (see section “Family Centered Care”). This multidisciplinary approach, with the patient’s best possible outcome as the foremost priority, helps eliminate traditional barriers such as “surgical versus medical,” “physician versus nursing,” and “parent versus caregiver.” The PCICU should have a physician leader who is a seasoned clinician possessing excellent skills in management, organization, and mentorship; these are essential qualities to maintain team cohesion and consistent excellent patient care (3). Managing of diverse viewpoints from many disciplines with patience, and consideration for all stakeholder contributions, is a hallmark of an effective leader.
Night and weekend physician coverage of the PCICU is an important concern. It is ideal to provide 24-hour in-hospital presence of an attending cardiac intensivist, to assist in dealing with complex management decisions, new admissions, and procedures. This may not be possible in all scenarios; provisions for rapid call-in of the attending physician must be in place in this case. Ideally also, the on call team of fellows, residents, and midlevel practitioners, as well as the attending physician, should have duties limited to the PCICU.
Special patient populations being admitted to the PCICU in increasing numbers include low birth weight (LBW) neonates, and adults with critical cardiac disease who are over the age of 18 years. The LBW patients require consultation with neonatologists to optimize management, especially with regard to pulmonary management of immature lungs, feeding and nutrition, infectious issues, and the patient with multiple congenital anomalies other than the heart defect. The adult with CHD greatly benefits from consultation with adult CHD specialists, who also assist in coordinating other adult consultants that may be needed such as neurologists and nephrologists. Another increasing patient group is patients requiring ECLS, in the form of ECMO, or ventricular assist devices (VADs). The complex problem of anticoagulation regimens to prevent thrombosis while not allowing excessive bleeding requires ready availability of consultants from hematology, or hematopathology services whose specialized knowledge of coagulation enhances care of these patients.
Relationship between the PCICU and the Cardiac OR and Catheterization Laboratory
PCICU care should be delivered as a continuum of care, and not start with the postcatheterization or postsurgical care (3,5). This should begin with the presurgical or catheterization admission to the PCICU, surgical or catheterization planning conference, or even the prenatal multidisciplinary conference where the care and disposition of antenatally diagnosed CHD patients is discussed. Ideally, patients requiring intensive care are admitted preoperatively to the same unit, under care of the same team as they will have postoperatively to facilitate better knowledge of the patient’s unique condition and communication with the surgeon, cardiac catheterization team, and family. Colocation of the PCICU near catheterization laboratories and cardiac operating rooms facilitates communication, rapid transport of very unstable patients, and proximity of skilled assistance during crises in the PCICU, operating rooms (ORs), or catheterization laboratory. The continuum of care principle also applies to immediate postoperative or postcatheterization care, with clear handoff communication from the procedural teams to the PCICU team, and continuing the support provided in the OR or catheterization laboratory.
Continuous Quality Improvement and Patient Safety in the CICU
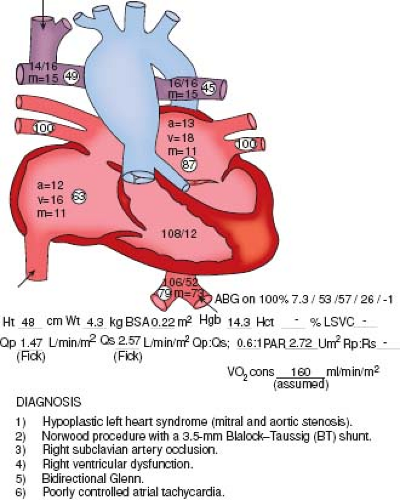
Improving patient safety, reducing medical error, and optimizing outcomes of care have been a priority of the U.S. health care system since the landmark publications of the Institute of Medicine, To Err is Human in 1999 (10), and Crossing the Quality Chasm in 2001 (11). These issues are particularly important in the PCICU, where complex pathophysiology, invasive procedures, multiple caregivers, multiple physician orders and medications, and unpredictable nature of events present a multitude of opportunities for errors, both active and latent, which may result in adverse events (12). A culture of patient safety involves change that begins with the PCICU leadership, and affects everyone involved in PCICU care, including junior physicians, nurses, and ancillary personnel. Important principles identified to promote a safety culture in complex environments include: leadership promoting a safety culture; process design based on human limitations that eliminates unnecessary variability; promoting team work and team functioning; proactive anticipation of unexpected events; and creation of a learning environment that empowers all personnel levels to report errors and to speak up when unsafe practices are encountered. Handoffs of care, whether between nursing or physician shifts, on transfer from operating room to PCICU or transition to a new care team, are frequent sources of error and miscommunication. The use of checklists improves consistency of communication and prevents many avoidable errors (13). Communication in the PCICU environment is a key concern; one simple method unique to this environment is to place a diagram of the cardiac anatomy of each patient, along with the recent surgical or catheterization procedure, on or near the bed, for easy visibility during an acute event (Fig. 24.1). Other programs with demonstrated effectiveness are central catheter insertion and maintenance “bundles” which include a checklist for insertion that guarantees full sterile precautions during insertion, placement of a chlorhexidine disc at the catheter insertion site, regular dressing changes, and a daily assessment of the need for the catheter. The PCICU patient is at high risk for central line–associated
blood stream infections (CLABSI) and coordinated efforts have resulted in a reduction in these events by as much as 80% to 90% (14,15).
blood stream infections (CLABSI) and coordinated efforts have resulted in a reduction in these events by as much as 80% to 90% (14,15).
The electronic medical record (EMR) is increasingly utilized in PCICUs and indeed will become a requirement in the United States in the coming years. The EMR can be an important factor in improving the quality of data recorded that can lead to improved patient outcomes. The EMR can be an important tool for legible, accessible documentation of physician admission and daily progress notes, as well as the electronic nursing PCICU flowsheet that documents not only bedside monitor data, but all medications and nursing interventions. The ability to perform bedside echocardiography efficiently, and to review the images themselves at the bedside, is important to optimize PCICU care. Computerized physician order entry can lead to more accurate and timely carrying out of orders, and decision support functions tied to hospital formulary can help avoid medication errors. Implementation of a complete EMR system is costly, and careful planning is needed to ensure that the EMR interfaces with all other electronic systems, such as picture archiving and communication (PACS) systems and cardiology databases that display echocardiographic and catheterization images. Processes of care must be understood, and the transition to EMR carefully planned to avoid work-arounds that prevent full and effective implementation of the system (16,17). Because of the complexities of care in the ICU setting, and the rapid proliferation of electronic data available to clinicians, in recent years there has been an increase in decision support analytic programs as part of the EMR, where real-time data are fed into algorithms for patient care (18). Even more complex is the field of streaming analytics for the ICU, where real-time physiologic data streams are combined with complex computer algorithms that rapidly analyze patterns and trends to provide early warning of impending deterioration (19,20). An example of streaming analytics utility is a recent report demonstrating that increased ST segment variability 4 hours prior to respiratory failure or cardiac arrest had significant predictive ability for these events in 21 postoperative hypoplastic left heart syndrome (HLHS) patients after the Norwood Stage I palliation (21).
The PCICU team must have processes in place for continuous quality improvement through local and collaborative databases, regular data and outcomes reviews, analysis of adverse events and complications, and mortality reviews. Databases available for PCICUs include the Virtual PICU Database Cardiac Module (22), Society of Thoracic Surgeons’ Congenital Heart Surgery Database, (23), Congenital Cardiac Anesthesia Society Database (24), and IMPACT Registry for catheter interventions (25). Recently, the Pediatric Cardiac Critical Care Consortium (PC4) was formed and consists of a comprehensive clinical registry to collect data on patient characteristics and outcomes for the PCICU population (26). PC4 also has the purpose of being a quality and outcomes collaborative learning organization to identify and disseminate best practices through continuous quality improvement efforts.
Family-Centered Care
Admission to the PCICU for an intervention or illness, whether planned or emergent, is a stressful and emotional experience for the patient, parents, and family. There has been considerable recent progress in involving the family as much as possible in their child’s care, with virtual abandonment of visitation policies, and active encouragement of the family to participate in rounds and aspects of their child’s care allowing the parent to be at the child’s bedside whenever possible (27). Comfortable family waiting areas in close proximity to the PCICU are also very desirable. Private conference rooms for discussions with the family, away from the unit, are another important feature to allow privacy. Newer ICU rooms incorporate enough space into individual patient rooms to accommodate parent sleeping
in the room. Allowing parents to be present on work rounds to hear the presentation and discussion of their child’s care has become an important routine in many PCICUs that, if done sensitively, increases the flow of accurate information to the parent and reduces stress. In a recent survey of PCICU physicians and nurses, 77% responded that parents had a right to be present during work rounds, 57% felt parents should be allowed to be present during invasive procedures such as tracheal intubation and arterial and central line placement, and 75% would allow them to be present during cardiopulmonary resuscitation. Of these scenarios, parents wanted to be present about 65% of the time for work rounds, but only about 30% of the time for invasive procedures, and for CPR (28). Family-centered care may require a change of culture among the physicians and nurses in the PCICU, and there must be an adequate orientation of the family members as to policies and expectations. With the presence of so many families in the PCICU, strict policies about disclosure of medical information heard about other patients’ conditions must be explained and enforced. Despite the occasional inconveniences or changes in well-established routines, adopting family centered care will result in greater parent and patient satisfaction and help to reduce the stress, uncertainty, and other negative emotions associated with PCICU admission.
in the room. Allowing parents to be present on work rounds to hear the presentation and discussion of their child’s care has become an important routine in many PCICUs that, if done sensitively, increases the flow of accurate information to the parent and reduces stress. In a recent survey of PCICU physicians and nurses, 77% responded that parents had a right to be present during work rounds, 57% felt parents should be allowed to be present during invasive procedures such as tracheal intubation and arterial and central line placement, and 75% would allow them to be present during cardiopulmonary resuscitation. Of these scenarios, parents wanted to be present about 65% of the time for work rounds, but only about 30% of the time for invasive procedures, and for CPR (28). Family-centered care may require a change of culture among the physicians and nurses in the PCICU, and there must be an adequate orientation of the family members as to policies and expectations. With the presence of so many families in the PCICU, strict policies about disclosure of medical information heard about other patients’ conditions must be explained and enforced. Despite the occasional inconveniences or changes in well-established routines, adopting family centered care will result in greater parent and patient satisfaction and help to reduce the stress, uncertainty, and other negative emotions associated with PCICU admission.
Evaluation and Management of the Cardiovascular System
The frequent or continual clinical assessment of cardiac output (CO) and oxygen delivery, and ensuring that they are optimized, or at least that measures are being undertaken to do so, are the central tenets of cardiac intensive care. This section will review the various methods to assess cardiac output.
Physical Examination, Bedside, and Laboratory Data
Despite the availability of many technologically advanced methods, cardiac output should always be assessed by inspection and physical examination in addition to monitoring data, and the invasive and noninvasive methods described below. Poor cardiac output is often heralded by pale or mottled and cool extremities, diminished peripheral pulses, delayed capillary refill (>2 to 3 seconds), increasing cyanosis, tachycardia, diminished pulses, poor heart tones, presence of third or fourth heart sounds, and evidence of hepatic and pulmonary congestion. Urine output is often decreased, acidosis may be present. In the presence of established low CO end-organ dysfunction may be apparent on laboratory testing. Lethargy or irritability may accompany the low CO in a conscious patient. Although these are simple principles, they should not be forgotten in the modern profusion of technology-based monitoring methods.
Routine continuous monitoring of physiologic variables in the PCICU including heart rate, electrocardiogram (ECG), arterial and central venous pressure waveforms, temperature, and end-tidal CO2 (ETCO2) can give important information about CO, including the ECG displaying nonsinus rhythm, elevated filling pressures or lack of an “a” wave on atrial filling pressures, acute decrease in ETCO2 with increased ETCO2 to PaCO2 gradient, blunted arterial waveform upstroke or excessive respiratory variation, and increased core-to-peripheral temperature gradient. Chest radiograph findings include cardiomegaly, pulmonary edema, or alternately, a paucity of pulmonary vascular markings associated with inadequate pulmonary blood flow. Laboratory data include an increasing calculated base deficit or serum lactate or persistent lactate above 2 mmol/L (29,30). Lactate clearance (normalization of serum lactate over time since admission after cardiac surgery to <2 mmol/L over <48 hours) was given a Class I recommendation (benefit outweighs risk, should be measured), evidence level B (limited populations, nonrandomized studies) by a PCICS task force evaluating monitoring modalities (31).
The cardiac biomarkers, B-type natriuretic peptide (BNP) and cardiac troponin, can be measured as markers of congestive heart failure/myocardial dysfunction (BNP) and myocardial cell injury (troponin) in critically ill patients in the PCICU setting, either postoperatively or in the setting of medical cardiac disease. With the caveat that troponin is elevated after any intracardiac surgery, troponin levels that do not decrease after surgery have been associated with higher risk of poor outcomes. The PCICS task force assigned a recommendation level of II (benefit outweighs risk, measurement may be considered) level of evidence B for measurement of BNP and/or troponin (32).
Echocardiography
Bedside echocardiography in the PCICU can be a very important tool for assessment of cardiovascular performance at a given point in time. Whether formal echocardiogram with complete anatomic and functional assessment, with official interpretation or reporting by an echocardiographer (preferred), or a rapid assessment with portable echocardiography during a crisis, echocardiography is indispensible as a frequent modality for evaluation of a patient with signs of a low CO in the PCICU, yielding information not obtainable by other methods. Besides the obvious measurement of ventricular function by qualitative assessment, ejection and/or shortening fraction, or myocardial performance index, many other important findings are made during low CO states. These include residual anatomic defects such as outflow tract obstruction, presence and degree of valvar regurgitation or stenosis, pericardial fluid or thrombus leading to a diagnosis of tamponade, assessment of right ventricular function, tricuspid regurgitation jet and position of the interventricular septum to assess pulmonary hypertension, and many others (33). If standard transthoracic views are not adequate for any reason, transesophageal echocardiography can be performed in the intubated, sedated patient (34). Bedside echocardiography is an excellent modality to define intracardiac anatomy; but extracardiac structures (systemic-pulmonary shunts, pulmonary veins, cavopulmonary anastomoses, aortic arch) are not as reliably imaged by echocardiography and may require additional imaging such as CT or cardiac catheterization (see section “Catheterization of ICU Patients”). The growing availability of portable, lightweight bedside ultrasound systems that have good image quality for cardiac structure and function has made this tool indispensible in modern critical care. The immediate availability of echocardiography, inclusion of bedside ultrasound in residency and fellowship curricula, and the ability to save images and echo clips have all contributed to the rapid increase in the use of this modality (35). A PCICS task force assessed the level of evidence for the use of bedside echocardiography in 2011, using the American Heart Association/American College of Cardiology evidence levels as a Class II recommendation, that is, the benefit of echocardiography outweighs any risk, but the level of evidence was deemed C, that is, only consensus opinion of experts, and case studies were available to support use of this modality (36).
Venous Oximetry
Measurement of true mixed venous oxygen saturation in the pulmonary artery of a patient with two ventricles and normal cardiac anatomy is rarely possible in the PCICU; much more practical is the measurement of oxygen saturation in the superior vena cava
(SVC), used as a surrogate for mixed venous saturation (37). Obviously this technique is used during cardiac catheterization for calculation of cardiac output using the Fick method. Many PCICU patients will have a catheter in the SVC for central venous access. A measured, not calculated, oxygen saturation, or ScvO2, can be an important trend monitor to assess cardiac output and oxygen delivery. This measurement can be made intermittently, or during changes in patient status, or in response to interventions.
(SVC), used as a surrogate for mixed venous saturation (37). Obviously this technique is used during cardiac catheterization for calculation of cardiac output using the Fick method. Many PCICU patients will have a catheter in the SVC for central venous access. A measured, not calculated, oxygen saturation, or ScvO2, can be an important trend monitor to assess cardiac output and oxygen delivery. This measurement can be made intermittently, or during changes in patient status, or in response to interventions.
Continuous monitoring of intravascular oxyhemoglobin saturation using reflectance catheters has been used in the umbilical artery, pulmonary artery, and adult-sized central venous catheters for a number of years, but only recently have standard pediatric-sized catheters become available for routine use to measure central venous oxygen saturation (ScvO2) in pediatric patients. In several small series of pediatric patients undergoing cardiac surgery, good correlation between ScvO2 as measured with the catheter versus blood co-oximetry (r2 = 0.8−0.9, bias −0.03 to +1.09% with standard deviation 4% to 8%) (38,39). The advantages of this method are that it is continuous, many patients have SVC catheters in place, and the oximetry catheters are the same diameter as standard central venous catheters. Although assessment of each patient must be individualized, ScvO2 below 50% in patients with univentricular hearts is often associated with low systemic oxygen delivery. For example, in univentricular neonates after Stage I palliation for HLHS, low ScvO2 was associated with death and need for ECMO. Goal-directed therapy targeting ScvO2 >50% is associated with high postoperative survival and low complication rate in this population (40). A PCICS task force evaluating venous oximetry classified the recommendations for use as Class I, benefit of using the modality significantly outweighs any risk and should be performed. The level of evidence was deemed B, limited populations evaluated and data derived from nonrandomized studies (41).
Near Infrared Spectroscopy
Near-infrared spectroscopy (NIRS) is a noninvasive optical technique used to monitor brain and somatic tissue oxygenation. Most devices utilize 2 to 4 wavelengths of infrared light at 700 to 1,000 nm, where oxygenated and deoxygenated hemoglobin have distinct absorption spectra. An oxygen saturation is derived using variants of the Beer–Lambert equation; this saturation is designated a regional oxygen saturation (rSO2), and is a weighted average of the saturation in venous, arterial, and capillary beds in the light path. Since its now classic description in 1977 by Jobsis (42), this technology has been the subject of over 1,000 publications, and because of its noninvasive compact, portable nature and potential to measure tissue oxygenation in the brain and other organ systems during surgery and critical illness, it is gaining more widespread clinical use.
Cerebral NIRS has been used to monitor adequacy of cerebral oxygen delivery and as a surrogate for adequacy of global oxygen delivery in patients recovering in the ICU after cardiac surgery and in patients on ECMO or VADs (43,44). Changes in rSO2 have a close correlation with changes in mixed venous saturation (SvO2) in both single and two-ventricle patients after congenital cardiac surgery (45,46).
NIRS can be used to measure tissue oxygenation in surgery and critical illness, and because of its noninvasive, continuous nature it has intuitive appeal for use in conditions where low cardiac output and other causes of shock would benefit from such continuous monitoring. Somatic NIRS using a probe placed on the flank at T10-L2 has been studied in a series of neonates after during and single-ventricle surgical palliation by Hoffman et al. (47). In nine neonates undergoing CPB with regional cerebral perfusion (RCP), mean cerebral rSO2 prebypass was 65% and somatic rSO2 59% and during RCP cerebral rSO2 81% versus 41% somatic rSO2, signifying relative tissue hypoxia due to lack of perfusion to subdiaphragmatic organs during this technique. After CPB, cerebral rSO2 decreased to 53%, but somatic rSO2 increased to 76% (35). In 79 postoperative neonates undergoing Norwood Stage I palliation for HLHS, a cerebral somatic rSO2 difference of <10% significantly increased the risk for biochemical shock, mortality, or other complications (48). Mean somatic rSO2 <70% was associated with a significantly increased risk of prolonged ICU stay, shock, and other complications.
Somatic NIRS has also been used to measure mesenteric rSO2 in neonates and infants after cardiac surgery, with a probe placed on the abdomen between the umbilicus and symphysis pubis. In a study of 20 patients, Kaufman et al. (49) compared mesenteric NIRS versus flank NIRS at T10-L2 to gastric pH measured by tonometry and lactate values. In 122 simultaneous measurements made in the first 48 hours after surgery, mesenteric rSO2 correlated significantly with gastric pH (r = 0.79), serum lactate (r = 0.77) and SvO2 (r = 0.89). These correlations were all better than those using flank NIRS. The authors concluded that mesenteric NIRS is a sensitive monitor of splanchnic tissue oxygenation, and may have utility at managing these patients and improving outcomes. In addition, for infants undergoing biventricular repair, flank (renal) rSO2 values below 50% for >2 hours in the immediate postoperative period were associated with higher peak creatinine levels, higher incidence of acute kidney injury, greater inotropic support, more days on mechanical ventilation, and higher serum lactate levels (50).
These studies lend credence to the idea that NIRS-directed targeted interventions could be utilized to improve oxygen delivery to tissues and organs, and potentially improve outcomes from surgery, anesthesia, and critical illness. Indeed, there are several nonrandomized cohort studies published demonstrating goal-directed therapies utilizing both cerebral and somatic NIRS-derived values, with good early clinical outcomes (51). To date, there is a lack of randomized controlled trials; and the increasingly widespread use of NIRS monitoring in the PCICU would appear to make it less likely that these studies will be performed. The strong physiologic rationale together with existing case series and cohort studies have provided sufficient evidence for many practitioners to adopt this monitoring as standard practice in these institutions. The PCICS task force evaluating NIRS concluded that its use constituted a Class II recommendation, with level of evidence B; the benefit of NIRS outweighed any risk and its use is reasonable and can be considered, but the level of evidence was derived from nonrandomized studies (52).
Thermodilution Cardiac Output
Percutaneous pulmonary artery (PA) catheterization has a limited role in the PCICU for several reasons. The small size of many patients precludes placement of adequate-sized sheaths and catheters, and many patients in whom PA catheter monitoring would be desirable have intracardiac shunting or valvar regurgitation, invalidating results of standard thermodilution cardiac output measurements and confusing mixed venous oxygen saturation (SvO2) measurements. In addition, frequent need for right-sided intracardiac surgery makes PA catheterization undesirable. Thus, when pulmonary artery pressure or SvO2 monitoring is indicated, transthoracic PA lines are the most common method in congenital heart surgery. The availability of continuous central venous oxygen saturation catheters (see section “Venous Oximetry”), and the perception that the risk: benefit ratio for PA catheter placement is most often unfavorable, limits the indications for this technique (53,54,55,56).
Cardiac index may be measured by standard thermodilution methods, with care taken to input the correct calculation constant into the monitor software according to the catheter size and length, and volume and temperature of injectate. The average of three consecutive injections made in rapid succession at the same point in the respiratory cycle, that is, expiration, will optimize conditions to achieve an accurate measurement during steady state conditions.
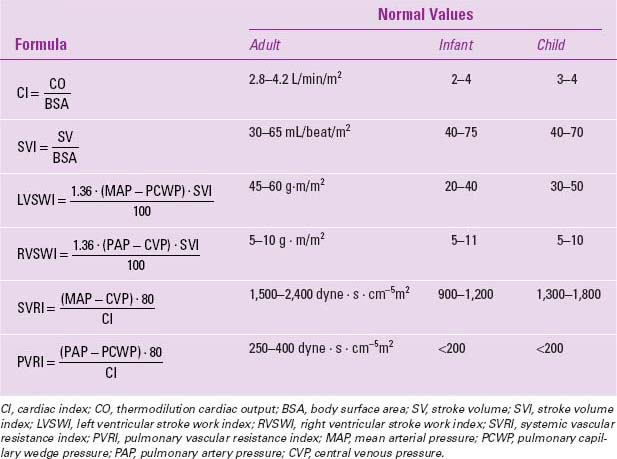
Vascular resistances and stroke volume can also be calculated, using the formulae in Table 24.1 (55,56).
Vascular resistances and stroke volume can also be calculated, using the formulae in Table 24.1 (55,56).
TABLE 24.1 Hemodynamic Formulae for Pulmonary Artery Catheter Monitoring | |
|---|---|
|
Hemodynamic data represent only a portion of the information available from an oximetric PA catheter. The other half consists of oxygen delivery and consumption measurements and calculations, which may also be used to guide therapy in the critically ill patient with LCOS (55,56) (Table 24.2). They require either measurement of mixed venous and systemic arterial saturations from blood samples from the tip of the PA catheter and arterial line (measured by co-oximetry, not calculated), or substitution of these values with SvO2 from the oximetric catheter (a valid assumption if properly calibrated), and pulse oximeter value instead of measured systemic saturation. There are data from adult and pediatric critical care literature suggesting that the ability to increase and maximize both oxygen delivery and consumption may improve outcome, and is a predictor of survival from critical illness, including postoperative cardiac surgery (57,58,59,60). The PCICS task force evaluating monitoring with a PA catheter for patients in low cardiac output and shock as a Class IIa recommendation, that is, the benefit of a PA catheter outweighs any risk, and it is reasonable to use this modality in patients where low CO continues despite standard therapies and use of less invasive monitors. The level of evidence, however, is C, limited populations studied consisting of case series, and consensus of experts (61).
Lithium Dilution and Pulse Contour Analysis Cardiac Output
Because traditional percutaneous, balloon-tipped pulmonary artery catheterization is limited in small children, and those with intracardiac shunting, several other recent methods to measure cardiac output and oxygen delivery in patients with CHD have been applied. Lithium dilution cardiac output (LiDCO) uses a standard central line in the SVC or even a peripheral IV catheter, and a special femoral artery catheter equipped with a lithium detecting electrode. A dilute solution of lithium chloride is injected into the vein, and arterial blood is withdrawn into the lithium electrode. The cardiac index is related to the area under the curve of the change of lithium concentration. This method has been demonstrated to have reasonable correlation with thermodilution cardiac output in children after congenital heart surgery. In a study of 48 measurements in 17 patients 2.6 to 34 kg, correlation between LiDCO and thermodilution cardiac output was good (r2 = 0.96), mean bias was −0.1 ± 0.31 L/min (62).
Transpulmonary thermodilution cardiac output uses a similar principle as LiDCO, with temperature as the indicator instead of lithium concentration. Cold saline is injected into a central venous catheter, and via a thermistor placed in a femoral artery, a time temperature curve is derived, which correlates reasonably well with standard thermodilution cardiac output as measured by a standard pulmonary artery catheter (63). Both lithium and any thermodilution method are limited to patients without any intracardiac shunting, significantly restricting their use in patients with CHD.
Yet another newer method is pulse contour analysis of the arterial waveform (PiCCO), which relates the contour and area under the curve to the stroke volume, and thus the cardiac output. This continuous method is periodically calibrated using the transpulmonary thermodilution cardiac output as described above (again making the method invalid with intracardiac shunting), and demonstrates a good correlation with transpulmonary thermodilution in a recent study of 24 pediatric patients after cardiac surgery (r2 = 0.86, mean bias 0.05 ± 0.4 L/min/m2) and in pediatric patients undergoing catheterization laboratory hemodynamic assessment after cardiac transplantation (64,65). A recent comprehensive review of publications of
this method in children and animal models of pediatric disease agree with the assessment that the stroke volumes and cardiac outputs derived with pulse contour analysis are generally accurate and precise, and may be a useful adjunct for the measurement of cardiac output in critically ill children (66). Pulse pressure variation as determined by the PiCCO method was a better predictor of intravascular fluid responsiveness in a series of 26 infants before and after CHD surgery; proving superior to echocardiographic-derived measures such as stroke volume index and conventional measure such as central venous pressure (67). The PCICS task force evaluating monitoring systems concluded that more data were required for both the LiDCO and PiCCO systems before recommendations could be made regarding their use (68).
this method in children and animal models of pediatric disease agree with the assessment that the stroke volumes and cardiac outputs derived with pulse contour analysis are generally accurate and precise, and may be a useful adjunct for the measurement of cardiac output in critically ill children (66). Pulse pressure variation as determined by the PiCCO method was a better predictor of intravascular fluid responsiveness in a series of 26 infants before and after CHD surgery; proving superior to echocardiographic-derived measures such as stroke volume index and conventional measure such as central venous pressure (67). The PCICS task force evaluating monitoring systems concluded that more data were required for both the LiDCO and PiCCO systems before recommendations could be made regarding their use (68).
TABLE 24.2 Oxygen Delivery and Consumption Formulae | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
A recent survey of 162 pediatric cardiologists, intensivists, and surgeons found that serial lactate monitoring was the monitoring strategy most used by respondents (94%) (69). Multisite NIRS was used in 67%, and the combination of lactates and NIRS in 78%. The PCICS has published an evidence-based review for monitoring of postoperative cardiac patients (70).
Table 24.3 summarizes physical examination findings and other data associated with low cardiac output.
Prevention and Management of Low Cardiac Output Syndrome
Determinants of Cardiac Output and Oxygen Delivery
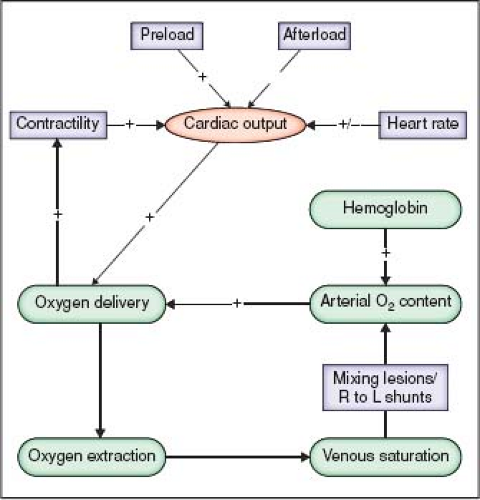
Besides the unique factors discussed earlier that alter the hemodynamic management in patients with CHD, it is important to recognize that alterations of preload, afterload, contractility, and heart rate are four cornerstones that affect cardiac output before and after surgery (Fig. 24.2). In addition, diastolic dysfunction is common in patients with CHD, particularly after right heart surgery, and measures to improve preload and possibly ventricular compliance are important. The oxygen carrying capacity of blood is improved by increasing the hemoglobin concentration. Each of these factors should be adjusted for the specific congenital cardiac lesion and the cardiovascular physiology that is associated
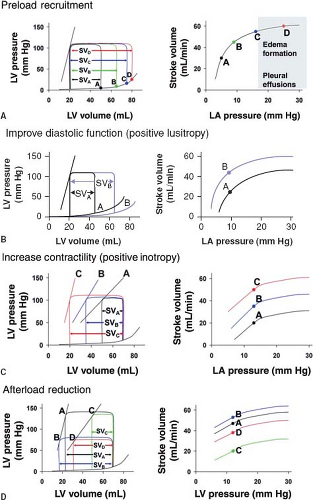
with the lesion. Figure 24.3 details expected changes in ventricular pressure–volume and Starling relationships with manipulation of preload, afterload, contractility, and lusitropy.
with the lesion. Figure 24.3 details expected changes in ventricular pressure–volume and Starling relationships with manipulation of preload, afterload, contractility, and lusitropy.
TABLE 24.3 Clinical Findings in Low Cardiac Output State | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pharmacologic Therapy for Congenital Heart Disease
The goal of drug therapy in an acute setting should be to optimize cardiac output and oxygen delivery; improve perfusion pressure to vital organs such as brain, heart, and kidneys and maintain an optimal balance between systemic and pulmonary blood flows with an appropriate level of oxygenation. High levels of inotropic and vasoactive infusions in the first 48 hours postoperatively for infants under 6 months of age are associated with poor outcomes: death, mechanical circulatory support, prolonged ventilation, neurologic injury, renal failure, and long ICU stays (71). Therefore, it is important to understand the actions of pharmacologic agents and to use them judiciously at the lowest effective doses.
The drugs that may be used in the acute hemodynamic management of patients can be categorized as belonging to one of these functional classes:
Inotropes (epinephrine, dopamine, dobutamine, milrinone, calcium)
Chronotropes (isoproterenol)
Vasoconstrictors (norepinephrine, phenylephrine, vasopressin, terlipressin)
Vasodilators (nitroglycerin, nitroprusside, prostaglandins, sildenafil, nitric oxide, fenoldopam, nicardipine, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers and nesiritide)
Beta adrenergic antagonists (esmolol)
Inodilators (milrinone, levosimendan)
The cardiac intensivist must keep in mind that the indications for and doses of these drugs in an individual patient are highly variable. Tables 24.4 and 24.5 summarize the effects and recommended dosages for these drugs. Affects such as age, disease state, and adrenergic receptor up or downregulation necessitate frequent titration of drugs to effect. Attempts to classify the level of vasoactive drug support have included the inotrope score, the vasoactive inotrope score (VIS), and most recently, the vasoactive inotrope score index (VISindex) (72). The VIS is a weighted score for the following vasoactive drugs: dopamine (mcg/kg/min) + dobutamine (mcg/kg/min) + (epinephrine (mcg/kg/min) × 100) + (milrinone (mcg/kg/min) × 10) + (vasopressin (units/kg/min) × 10,000) + (norepinephrine (mcg/kg/min) × 100). Adding the maximum VIS, and the duration of vasopressor/inotropic support yields the VISindex, with scores ranging from 1 (low support) to 6 (high support). An overall high VISindex ≥3 was strongly associated with individual and composite poor outcomes in 244 infants undergoing CHD surgery.
Inotropes
Epinephrine
Epinephrine is an endogenous catecholamine that is secreted by the adrenal glands and has strong alpha- and beta-adrenergic receptor activation. This action on both types of adrenergic receptors leads to the complexity of response in different organs and tissue beds. The response of exogenously administered epinephrine depends on the ratio of alpha- to beta-receptors in the individual tissue beds as well as to the dose of epinephrine given. At lower doses (<0.05 mcg/kg/min), epinephrine causes a moderate increase in systolic blood pressure that is mainly due to increased ventricular contractility (73). Activation of the β2-receptors in the vasculature of the skeletal muscles usually leads to a decrease in the systemic vascular resistance and the diastolic pressure. As the dose is progressively increased, more prominent peripheral vasoconstriction is seen due to the activation of the α-receptors in other vascular beds (74). Renal blood flow is consistently decreased as vascular resistance in all segments of the renal vasculature increases (75). Epinephrine is often used as a strong inotrope in the support of the failing myocardium. Epinephrine’s action on the predominant β1-receptors in the heart leads to an increase in contractility and heart rate. Higher doses will lead to a decrease in the refractory period of the AV node and an increase in the automaticity of the myocardium, which may predispose to the development of atrial or ventricular arrhythmias.
TABLE 24.4 Inotropic Drugs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 24.5 Vasoactive Drugs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
During cardiopulmonary resuscitation, epinephrine is the vasopressor of choice since it has profound α-adrenergic stimulation that aids in maintaining the cerebral and coronary perfusion pressure during cardiovascular collapse (76). The American Heart Association recommended dose of epinephrine in children for bradycardia, asystolic or pulseless arrest is 0.01 mg/kg intravenously, repeated at 3- to 5-minute intervals. Higher doses of epinephrine are no longer recommended (77,78).
Epinephrine is used as an infusion primarily in the dose range from 0.02 to 0.2 mcg/kg/min, although doses up to 0.5 mcg/kg/min or higher are occasionally required in the short term for acute severe low cardiac output in situations such as weaning from bypass, or during ECMO cannulation or emergency institution of bypass. Prolonged exposure to high doses of epinephrine, that is, 0.1 to 0.2 mcg/kg/min or higher for more than several hours, may cause myocardial necrosis in infants, and strong consideration should be given for mechanical circulatory support in this instance (79).
Dopamine
Dopamine is a naturally occurring catecholamine that is an immediate precursor of norepinephrine. Most of the functions of endogenously excreted dopamine are as a central neurotransmitter, though it has been found in the peripheral circulation as well. The cardiovascular effects of exogenously administered dopamine are due to the activation of a variety of receptors that have different affinity for the drug (80). At a lower dose (<5 mcg/kg/min), the primary receptors that are activated are the dopaminergic-1 (DA-1) receptors present in the renal, mesenteric, and coronary vascular beds. Infusion of low-dose dopamine can lead to an increase in renal blood flow and an increase in glomerular filtration rate (GFR) (81). However, “renal dose” dopamine has not been demonstrated to have direct beneficial effects in improving renal function (82). As the dose of the drug is increased, stimulation of the β1-receptors in the myocardium has inotropic and chronotropic effects (83). At these doses, dopamine causes an increase in cardiac output, decrease in pulmonary capillary wedge pressure, and there is usually a decrease in systemic vascular resistance with only slight changes in blood pressure. The increase in heart rate is much less than for isoproterenol. Total peripheral resistance is usually unchanged with low or intermediate doses of dopamine, due to vasodilatory action of dopamine on regional vascular beds. At higher doses (>10 mcg/kg/min), more α1-receptors are activated leading to a more intense peripheral vasoconstriction and an increase in vascular resistance. Dopamine causes release of norepinephrine from nerve endings; this also adds to its pharmacologic effect of adrenergic stimulation.
The volume of distribution and the clearance of dopamine are highly variable, underscoring the principle of titrating this drug to effect in the individual patient (84). Dopamine in the dose range 5 to 15 mcg/kg/min is commonly used as an inotropic support to assist in the weaning from cardiopulmonary bypass, and in the early postoperative period. In recent years some practitioners have avoided dopamine because of its role as a neurotransmitter, which can cross the blood–brain barrier and is known to suppress pituitary function, particularly thyroid releasing hormone, in infants and children (85). This potential adverse effect is not seen with other natural or synthetic catecholamines (86).
Dobutamine
A synthetic congener of dopamine, dobutamine’s pharmacologic actions are due to its activation of α– and β-adrenergic receptors. Dobutamine has not been shown to have any effect on the dopaminergic receptors or lead to the release of norepinephrine from nerve endings. The primary action of dobutamine is on β1-receptors with only a small effect on β2– or α1-receptors. This action causes increased inotropy and chronotropy. Cardiac output is markedly enhanced and the left-sided filling pressures are decreased. Total peripheral resistance is unchanged or may decrease with the use
of dobutamine. This effect may be especially beneficial in treating patients with ventricular dysfunction.
of dobutamine. This effect may be especially beneficial in treating patients with ventricular dysfunction.
There is little direct increase in renal blood flow as is seen with dopamine. Dobutamine has been shown to be effective in improving depressed cardiac index after cardiopulmonary bypass in children with CHD in doses ranging from 5 to 15 mcg/kg/min (87). Comparison with newer inotropic drugs such as milrinone demonstrates similar improvements in stroke volume but a more profound decrease in left ventricular filling pressures and vascular resistance than with the phosphodiesterase inhibitors (88). Increased heart rate is more prominent with dobutamine than with milrinone. At equivalent inotropic doses dobutamine enhances the automaticity of the sinoatrial (SA) node to a much less extent than isoproterenol (89). Higher doses of dobutamine (>15 mcg/kg/min) can predispose to the development of atrial or ventricular arrhythmias. Despite the fact that dobutamine will increase cardiac output in neonates by increasing stroke volume, heart rate, and cardiac output, it is frequently used to stress the myocardium, will increase myocardial oxygen consumption and will produce diastolic dysfunction in some patients with repaired CHD (90,91).
Milrinone
Milrinone is a bipyridine derivative that induces vasodilation and exerts a positive inotropic effect by inhibiting phosphodiesterase III. This leads to the accumulation of cyclic adenosine monophosphate (cAMP), independent of adrenergic receptor stimulation (92). The increase in cAMP in cardiac myocytes improves systolic and diastolic function by altering calcium influx (93) and by altering uptake and binding of calcium to myofilaments; whereas in vascular smooth muscle, accumulation of cAMP predominantly effects the removal of calcium across sarcolemma and therefore causes vasodilation. The decrease in SVR allows phosphodiesterase inhibitors to increase cardiac output and oxygen delivery without increasing myocardial work and oxygen demand. Because of the dual effects on the inotropic state of the heart and the vascular resistance, milrinone has been used extensively in the treatment of congestive heart failure, pulmonary hypertension, and postoperative low cardiac output.
Milrinone has been shown to be an effective inotrope in adults as well as children with CHD (94,95). Peripheral vasodilation also ensues as a result of vascular smooth muscle relaxation. Chang et al. reported that milrinone (loading 50 mcg/kg followed by an infusion of 0.5 mcg/kg/min), when administered to neonates with low cardiac output after cardiac surgery, was able to lower filling pressures, systemic and pulmonary vascular resistances (>25%), and improve cardiac index (from 2.1 to 3.1 L/min/m2) (96). Bailey et al. found an increase in cardiac output of 18% after milrinone therapy in 20 children undergoing corrective surgery for congenital cardiac defects (97). Milrinone also improves diastolic function. Hypotension and reflex tachycardia may result as a side effect of milrinone therapy. Mehra et al. reported a 4% incidence of thrombocytopenia in 71 patients who received long-term intravenous milrinone therapy (>3 days) (98). Milrinone is primarily renally excreted and higher bolus doses (50 to 75 mcg/kg) may show prolonged hemodynamic effects in patients with impaired renal function. Serum half-life was found to be 0.8 hours in patients with congestive heart failure (99). Milrinone has also been suggested to have a higher volume of distribution and a faster clearance in infants and children as compared to adults (99). The dose recommended for milrinone therapy in patients with normal renal function is a bolus of 50 mcg/kg followed by an infusion of 0.25 to 0.75 mcg/kg/min. Hypotension seen with a loading dose may be avoided by reducing or eliminating the loading dose and simply beginning the infusion, recognizing that therapeutic plasma levels will not be achieved for several hours.
In recent years milrinone has gained widespread use in CICUs, and is one of the few regimens that has been subjected to prospective, randomized, double blind, controlled study. Hoffman et al. studied 227 infants and children undergoing cardiac surgery with bypass; high-dose milrinone (75 mcg/kg loading dose after bypass, followed by 0.75 mcg/kg/min infusion) reduced the incidence of LCOS by 55% compared to placebo or low-dose milrinone (25 mcg/kg load and 0.25 mcg/kg/min) (100). In a pharmacokinetic study of 16 neonates undergoing Norwood Stage I palliation, a loading dose of 100 mcg/kg into the bypass circuit at the start of rewarming provided therapeutic plasma concentrations, but an infusion of 0.5 mcg/kg/min caused a significant increase in plasma milrinone concentration over the first 12 hours; impaired renal function was thought to be the cause and neonates may require lower doses of 0.2 mcg/kg/min (101).
Calcium
The calcium ion is an integral part of the excitation–contraction coupling and impulse generation in myocardial cells and is a major determinant of vascular smooth muscle tone. Particularly in neonates, where the sarcoplasmic reticulum is not well developed, and the sequestration and release of calcium is thus inefficient, an adequate ionized calcium concentration is important to optimize myocardial contractility. Administration of calcium in the form of calcium chloride or calcium gluconate helps improve the inotropic function of the heart in the presence of hypocalcemia (102). Calcium functions primarily as a vasoconstrictor when the serum ionized calcium levels are normal. Routine administration of calcium salts upon termination of CPB is a subject of debate. The incidence of hypocalcemia during CPB is relatively high, but the ionized calcium levels usually are corrected to normal levels as weaning from CPB is attempted, (103) therefore calcium administration may not be required for most patients. Moreover, increasing evidence suggests that elevated intracellular calcium levels are associated with cell death and injury during ischemia and reperfusion injury (104). Murdoch et al. reported an increase in the SVRI (885 to 1,070 dyne·s/cm−5/m2) and a decrease in CI (4.44 to 3.85 L/min/m2) after administration of 10 mg/kg of CaCl2 in 12 children following cardiac surgery (105). A higher and more predictable amount of elemental calcium is available from the intravenous administration of calcium chloride than calcium gluconate or gluceptate (106).
The routine use of calcium as a cardiotonic agent early after bypass in the presence of normocalcemia is not well supported. Indeed there is good evidence from resuscitation literature and in adults that boluses of calcium or supranormal levels of calcium are associated with adverse outcomes. Moreover, it was recently shown that mortality after cardiac surgery with bypass was correlated with higher Ca2+ supplementation in infants aged under 1 year of age, suggesting that this agent should be used only with documented hypocalcemia with associated myocardial dysfunction, or in neonates receiving significant amounts of citrated blood products, and discontinued as soon as possible in the CICU (107). Calcium administration is not recommended in bradyasystoles unless severe hypocalcemia or hyperkalemia coexists or if the arrest is secondary to calcium channel antagonist drugs (77,78).
Chronotropes
Isoproterenol
Isoproterenol is a potent nonselective β-adrenergic agonist with only very minimal actions on α-receptors. Due to its vasodilatory β2-stimulatory actions as well as lack of α-receptor stimulation, isoproterenol leads to lowering of peripheral vascular resistance (73,108). Its vasodilatory actions may be seen in renal, mesenteric, and
pulmonary vascular beds. An intravenous infusion of isoproterenol has more chronotropic than inotropic effect, as opposed to dopamine or dobutamine. Myocardial oxygen demands are greatly exacerbated by isoproterenol and this may exacerbate or induce ischemia (109). Higher doses of isoproterenol can be arrhythmogenic and may induce ventricular tachycardia or fibrillation. This agent is contraindicated in dynamic obstruction of the right or left outflow tracts.
pulmonary vascular beds. An intravenous infusion of isoproterenol has more chronotropic than inotropic effect, as opposed to dopamine or dobutamine. Myocardial oxygen demands are greatly exacerbated by isoproterenol and this may exacerbate or induce ischemia (109). Higher doses of isoproterenol can be arrhythmogenic and may induce ventricular tachycardia or fibrillation. This agent is contraindicated in dynamic obstruction of the right or left outflow tracts.
Vasoconstrictors
Norepinephrine
Norepinephrine is an endogenous catecholamine that is primarily released by the postganglionic adrenergic nerve endings. Besides being a major source of epinephrine, the adrenal medulla also contains norepinephrine in a smaller fraction (10% to 20%).
The actions of norepinephrine are very similar to epinephrine on the heart with strong stimulation of the β1-receptors and increase in myocardial contractility (75). There is a substantial difference in the peripheral action of the two drugs (73) and these differences account for the differences in their clinical use. Norepinephrine is a potent α1-agonist at all doses with minimal effects on the vasodilatory β2-receptors (73). As a result, even low doses of norepinephrine lead to an increase in the systolic and diastolic blood pressure. Systemic vascular resistance is increased as a result of the vasoconstriction of most peripheral vascular beds. Cardiac output is usually decreased or unchanged, depending upon the increase in total peripheral resistance. Heart rate may be slowed as a result of reflex increase in vagal tone, or may occasionally increase if the β1 effects predominate in an individual patient. Both of the endogenous catecholamines, epinephrine and norepinephrine can lead to hyperglycemia with prolonged infusions (110). Norepinephrine usually causes these effects at much higher doses than epinephrine.
Norepinephrine functions as a strong vasoconstrictor and is useful in the clinical situation of decreased systemic vascular resistance; or vasoplegic shock (111). The typical dose range of norepinephrine infusion varies from 0.01 to 0.2 mcg/kg/min.
Phenylephrine
Phenylephrine is a pure peripheral α1-receptor agonist used as a bolus or infusion where low systemic blood pressure must be treated acutely. The pure α-effects often result in reflex slowing of the heart rate, although this is not as pronounced in young infants. Its principal use in CHD is to acutely raise SVR when either ventricle is compromised by outflow obstruction, for example, tetralogy of Fallot (TOF) with low SVR leading to increased right to left intracardiac shunting and cyanosis during a “Tet Spell,” (112) and hypertrophic cardiomyopathy (113) or other left-sided lesions where the gradient across the obstruction is increased by low SVR. It is also useful in similar physiologies where an acute increase in SVR is needed to improve oxygenation, that is, partially obstructed systemic to pulmonary shunt or single-ventricle patient with pulmonic stenosis. Infusions can be used when frequent boluses are necessary, such as in the TOF patient with continuous spelling before transfer to the operating room. Phenylephrine is very effective at increasing the blood pressure, but its principle adverse effect is vasoconstriction of peripheral tissue beds, including skeletal muscle, skin, renal, and mesenteric. This vasoconstriction may be intense, and theoretically may compromise end-organ blood flow and function, leading many practitioners to limit its use to extreme situations. Extravasation of phenylephrine into the skin and subcutaneous tissues may lead to ischemia, necrosis, and tissue loss. A reflex slowing of heart rate may be observed, and vigilance is necessary with large phenylephrine doses.
Bolus dosing of phenylephrine is 0.5 to 5 mcg/kg or higher, and infusion dosing ranges from 0.02 to 0.3 mcg/kg/min, through a central venous catheter if possible.
Vasopressin
Vasopressin is a neurogenic polypeptide produced by the paraventricular nucleus of the midbrain in response to low blood pressure and is secreted by the posterior lobe of the pituitary. Vasopressin produces intense vasoconstriction and has an antidiuretic effect. Vasopressin exerts these effects via V1 (vasoconstriction) and V2 receptors (antidiuresis). In the past the most common use of vasopressin was to treat gastrointestinal bleeding. More recently vasopressin has been used as an alternative to epinephrine in the acute resuscitation; however, the superiority of vasopressin over epinephrine for this indication is not clear. A theoretical advantage of vasopressin is that it does not rely on adrenergic receptors, which may be downregulated in chronically elevated catecholamine states. In conditions of metabolic acidosis signal transmission via adrenergic receptors is also ineffective. Some conditions producing low blood pressure (i.e., septic shock) are associated with low plasma vasopressin concentration, suggesting inappropriately low vasopressin secretion. In some of these patients, hypersensitivity to the administration of vasopressin has been described, possibly due to upregulation of vasopressin receptors, and is in agreement with studies showing rapid desensitization to vasopressin (114).
Rosenzweig et al. (115) reported their experience with use of vasopressin in moribund pediatric patients after cardiac surgery. These patients were classified as unresponsive to standard vasopressors, and the dosage of vasopressin varied from 0.018 to 0.12 units/kg/hr. These doses of vasopressin produced an average increase in systolic blood pressure of 22 mm Hg (65 to 87 mm Hg). Plasma vasopressin levels were low in the three patients with measurements. Patients who had low blood pressure and poor cardiac function before the initiation of vasopressin therapy died. Vasopressin is particularly useful in cases of low SVR induced by excessive α-adrenergic blockade, such as with phenoxybenzamine or phentolamine (116). There are now a number of case series of vasopressin, and its synthetic analogue, terlipressin, being used to treat refractory vasodilatory shock in pediatric patients with cardiogenic and septic etiologies (117,118). Vasopressin doses range from 0.01 to a maximum of 0.05 units/kg/hr, and should be weaned and discontinued as soon as possible. In a recent retrospective review of 34 postoperative cardiac surgery patients, only 50% of the children responded to vasopressin with an increase in mean arterial pressure and a decrease in catecholamine dose (119). Hyponatremia is often seen with vasopressin infusions of several days’ duration, and serum sodium should be measured at least daily. A recent case series in 29 patients reported hyponatremia in 48% of patients receiving vasopressin but only 17% of patients not receiving the drug (p = 0.004) (120).
Vasodilators
Nitroglycerin
Nitroglycerin produces vasodilation by releasing nitric oxide (NO). The release of NO from nitroglycerin, unlike that of some other nitric oxide donors, is enzymatically mediated. Nitroglycerin is frequently referred to as a venodilator or coronary dilator, while sodium nitroprusside (SNP) is thought of as a preferential dilator of arteries, although these differences are difficult to demonstrate. The major indications for the use of nitroglycerin are myocardial
ischemia, hypertension, volume overload, congestive heart failure, and pulmonary edema. Venodilation associated with nitroglycerin therapy leads to a decrease in preload, with subsequent lowering of the left ventricular end-diastolic volume and pressure, and therefore decreased wall stress. The net effect is usually an improvement in the ratio of myocardial oxygen demand to delivery. Nitroglycerin also dilates both diseased and normal coronary arteries (121). Hypotension and reflex tachycardia are the potentially undesirable side effects. Nitroglycerin is used in the cardiac surgical patients for the treatment of systemic or pulmonary hypertension as well as to decrease filling pressure and improve cardiac index. In a study including 20 pediatric patients with CHD, of whom 14 had preoperative pulmonary hypertension, nitroglycerin (>2 mcg/kg/min) reduced both systemic and pulmonary vascular resistance (122). Improved cardiac index was seen only with higher doses. The authors suggest that the effect of the drug on the systemic and pulmonary arteries and on capacitance vessels is dose related. In lower doses (<2 mcg/kg/min), nitroglycerin mainly produced venodilation, as evidenced by an increased requirement of volume to maintain a constant right and left atrial pressure. The usual doses of intravenous nitroglycerin infusion are 0.5 to 5 mcg/kg/min.
ischemia, hypertension, volume overload, congestive heart failure, and pulmonary edema. Venodilation associated with nitroglycerin therapy leads to a decrease in preload, with subsequent lowering of the left ventricular end-diastolic volume and pressure, and therefore decreased wall stress. The net effect is usually an improvement in the ratio of myocardial oxygen demand to delivery. Nitroglycerin also dilates both diseased and normal coronary arteries (121). Hypotension and reflex tachycardia are the potentially undesirable side effects. Nitroglycerin is used in the cardiac surgical patients for the treatment of systemic or pulmonary hypertension as well as to decrease filling pressure and improve cardiac index. In a study including 20 pediatric patients with CHD, of whom 14 had preoperative pulmonary hypertension, nitroglycerin (>2 mcg/kg/min) reduced both systemic and pulmonary vascular resistance (122). Improved cardiac index was seen only with higher doses. The authors suggest that the effect of the drug on the systemic and pulmonary arteries and on capacitance vessels is dose related. In lower doses (<2 mcg/kg/min), nitroglycerin mainly produced venodilation, as evidenced by an increased requirement of volume to maintain a constant right and left atrial pressure. The usual doses of intravenous nitroglycerin infusion are 0.5 to 5 mcg/kg/min.
Sodium Nitroprusside
The hypotensive properties of sodium nitroprusside (SNP) were described in the late 1800s, however, the drug was not approved for clinical use until 1974. Frequently, nitroprusside is incorrectly referred to as a “direct, preferential arterial vasodilator.” Nitroprusside dilates both arteries and veins by releasing NO in an interaction with tissue compounds containing sulfhydryl groups. The released NO activates soluble guanylyl cyclase that increases cGMP. Nitroprusside is most commonly used to control blood pressure in hypertensive patients, and to decrease systemic vascular resistance thereby improving forward flow in patients with poor LV function or regurgitant lesions (mitral or aortic regurgitation). Because of its short half-life SNP allows precise control of blood pressure and SVR. In patients with diminished myocardial function, cardiac output is increased from an increased stroke volume as a result of decreased aortic impedance. Despite significant reductions in SVR, the blood pressure decrease is usually modest since an increase in cardiac output compensates for the decrease in SVR. The drop in blood pressure is more dramatic in patients with pre-existing hypovolemia or obstructive cardiac lesions. In patients with hypertrophic cardiomyopathy SNP may increase outflow obstruction, and patients with aortic or mitral stenosis may not be able to compensate with an increase in cardiac output, resulting in profound hypotension. SNP has been used for early postoperative afterload reduction after Norwood Stage I palliation to lower SVR and decrease Qp: Qs, with excellent early hemodynamics and high early survival rate (123).
One of the dangers associated with the use of nitroprusside use is toxicity from the formation of cyanide. Cyanide, a byproduct of SNP metabolism, is taken up by red cells and inactivated predominantly in the liver by reacting with thiosulfate. This reaction is catalyzed by the enzyme rhodanese; patients with liver failure are more susceptible to cyanide toxicity. If cyanide toxicity occurs, SNP should be stopped immediately, and after confirmation of diagnosis, the patient should be treated with 3% sodium nitrate followed by the administration of sodium thiosulfate. SNP should be used cautiously in patients with renal failure since they may have difficulty metabolizing the thiocyanate produced during breakdown of SNP. In a retrospective review of 63 children after cardiac surgery in whom SNP was used to control blood pressure or to lower systemic vascular resistance for hemodynamic purposes, 11% of patients experienced an elevated cyanide concentration. Mean SNP dose was 2.8 mcg/kg/min in those patients with elevated cyanide levels, and 1.1 mcg/kg/min for those without elevated levels; with an increased risk of elevated cyanide levels starting at 1.8 mcg/kg/min (124).
The starting dose of SNP is 0.5 to 1 mcg/kg/min and the dose can be titrated up to 5 mcg/kg/min. The higher doses pose greater risk for toxicity, so doses exceeding 3 mcg/kg/min should not be administered for longer than several hours. Sodium thiosulfate can be added to the infusion to eliminate cyanide; but alternative methods of hypertension treatment should be instituted instead. In a multicenter study of 118 infants and children receiving esmolol for blood pressure control on admission to the ICU after coarctation repair via thoracotomy; only 15% to 20% of neonates required SNP, 50% of patients aged 1 to 24 months, and 80% of patients aged 2 to 6 years required SNP for blood pressure control. The median maximal SNP dose was 3 mcg/kg/min, and there was no mortality, neurologic complication, or significant acidosis (125).
Prostaglandins
Prostaglandins such as PGE2 and PGI2 are the main metabolites of the arachidonic acid pathway. In the vascular tissues they are predominantly generated and subsequently released by the endothelium to bind to specific receptors on the underlying smooth muscle cells. This leads to the activation of adenylate cyclase and an increase in cAMP levels, which lowers intracellular Ca2+ and produces vascular smooth muscle relaxation.
PGE1, a synthetic PG preparation, is used to relax smooth muscle and temporarily maintain the patency of the ductus arteriosus in neonates with a duct-dependent systemic or pulmonary circulation. PGE1 when administered to 27 neonates in whom pulmonary or systemic blood flow was entirely or significantly dependent upon ductal patency, led to an improvement in hypoxemia and acidemia, as well as ductus dilation (126). It has been demonstrated to maintain ductal patency for as long as 2 months (127) and to reopen a recently closed ductus. Preoperative drug therapy with PGE1 has lowered the mortality and allowed planned surgeries, rather than desperate attempts at emergency palliation, which was frequently the case in the past. Most common side effects of PGE1 therapy are hypotension, apnea, hyperpyrexia and jitteriness are most commonly seen at higher doses (0.05 to 0.1 mcg/kg/min). These are usually reversible upon lowering the dose or discontinuation of the drug.
Besides management of neonatal CHD, PGE1 has been used to treat pulmonary hypertension secondary to mitral valve disease, (128) after congenital cardiac surgery (129) and after heart transplantation (130). There has been only limited research conducted in the use of inhaled PGE1.
PGI2 or prostacyclin (epoprostenol) is a relatively recent addition to the drug therapy and is a pulmonary vasodilator used in the management of primary pulmonary hypertension or pulmonary hypertension in the setting of CHD. This is most typically used in patients with severe, end-stage disease, including those awaiting lung transplantation, though it has been used as short-term therapy early after cardiac surgery (131). Systemic PGI2 is not entirely selective for the pulmonary vasculature and therefore results in systemic vasodilation and lowering of the systemic arterial pressure. PGI2 is spontaneously hydrolyzed to 6-keto-prostaglandin F1α with a half-life of 1 to 3 minutes. Due to its short half-life, aerosolized PGI2 (iloprost) like nitric oxide can selectively dilate pulmonary vessels with minimal effects on the systemic arterial pressure. Several anecdotal reports and a few small clinical studies suggest that inhaled PGI2 can reduce elevated pulmonary artery pressures and pulmonary vascular resistance (PVR). Schulze-Neick et al. (132) reported inhaled PGI2 and nitric oxide to have similar advantageous effects on reducing PVR in patients with CHD after cardiac surgery. Another study demonstrated reduction of pulmonary artery pressures and improvement in the right ventricular function following inhaled PGI2 therapy with bolus dosing (2.5, 5, 10 mcg) in nine patients undergoing cardiac surgery including heart transplantation (133).
The optimal dosing of epoprostenol remains undefined and dosing of inhaled PGI2 ranging from 1 to 50 ng/kg/min have been shown to be efficacious (134). This agent has also been demonstrated to lower elevated PAP and PVR in infants after congenital heart surgery with bypass (135).
The optimal dosing of epoprostenol remains undefined and dosing of inhaled PGI2 ranging from 1 to 50 ng/kg/min have been shown to be efficacious (134). This agent has also been demonstrated to lower elevated PAP and PVR in infants after congenital heart surgery with bypass (135).
Sildenafil
Sildenafil is a phosphodiesterase-5 inhibitor, which in its intravenous form, appears to be a selective and highly effective pulmonary vasodilator in a piglet model of meconium aspiration with severe pulmonary hypertension (136). Sildenafil has been shown in case reports to ameliorate the effects of nitric oxide withdrawal in a patient after cardiac surgery with persistent pulmonary hypertension (137). In doses of 0.5 to 2.0 mg/kg given NG, sildenafil was effective at lowering mean PAP in infants after atrioventricular septal defect repairs (138). Other small retrospective reviews demonstrate safety, and lowering of PA pressures as well as some evidence for shorter ventilation times and ICU stays if oral sildenafil is administered pre- and/or postoperatively in children with moderate to severe pulmonary hypertension (139,140).
Inhaled Nitric Oxide
A ubiquitous compound in the human body, nitric oxide is produced as a result of the conversion of the amino acid arginine to citrulline, a reaction that is facilitated by the enzyme nitric oxide synthase (NOS). Nitric oxide, being a very small and lipophilic molecule diffuses into the underlying smooth muscle cells producing an increase in intracellular cGMP levels and subsequent vasodilation (141). Inhaled nitric oxide (iNO) is highly selective for ventilated lung regions. It crosses the alveolar-capillary membrane leading to pulmonary vasodilation and a decrease in PVR. High affinity binding and immediate inactivation of inhaled nitric oxide (iNO) activity by hemoglobin limits the action of the drug to the pulmonary circulation. Inhaled nitric oxide therapy has been shown to be useful in the treatment of pulmonary hypertension, which is frequently seen in CHD (141,142). Pulmonary hypertension in patients with CHD is multifactorial, due to chronic hypoxemia or due to chronic elevation of pulmonary blood flow and/or pulmonary venous pressures. PVR is also increased immediately after cardiopulmonary bypass due to the endothelial dysfunction. Inhaled nitric oxide is ideally suited in selectively reducing PVR in this critical period (142). The pulmonary vascular selectivity of iNO may be especially useful in reducing the right ventricular afterload in patients undergoing heart transplant, where the donor hearts may not be accustomed to high PVR of that is usually seen in these patients (143). Several investigators have reported the use of iNO in the preoperative period as a test of reversibility of PVR and in predicting post CPB pulmonary hypertension, as well as using the preoperative evaluation to predict the use of the drug in the immediate post CPB period (141,144,145,146).
Others have reported the benefit of iNO in improving oxygenation, likely by improving pulmonary blood flow and ventilation–perfusion balance in congenital cardiac surgery patients (147). Even though iNO has been shown to be effective in a variety of congenital heart lesions, it may not be helpful in reducing PVR in all patients. In a double blind study, the combination of milrinone 0.5 mcg/kg/min and NO 30 ppm was more effective at lowering PAP than either agent alone (148). The nonresponders are usually those who have long standing pulmonary hypertension and extensive remodeling of the pulmonary vasculature. NO also has not been shown to improve postoperative outcome, the cost is prohibitive at several thousand dollars per day of use, the delivery system setup is complex, as is transport with nitric oxide, and there are toxicity issues (see below in this section). It is therefore recommended that iNO be used as a last resort in a patient with known or suspected severe pulmonary hypertension resulting in low cardiac output (in two-ventricle patients), or severe desaturation due to reduced pulmonary blood flow in single-ventricle patients with cavopulmonary connections. Importantly, iNO is ineffective in patients with obstructions to pulmonary flow, and may in fact be detrimental in patients with obstruction to pulmonary venous drainage or left ventricular inflow.
Inhaled nitric oxide can be administered using a face mask or via nasal cannulae in a spontaneously ventilating patient, or added to the inspiratory limb of the breathing circuit in a mechanically ventilated patient. The most commonly used dose range is 10 to 20 ppm, though a decrease in PVR has been demonstrated with doses as low as 2 to 5 ppm. Side effects of inhaled nitric oxide at these doses are minimal, even with prolonged therapy. Some have advocated the use of iNO in the sweep gas of the CPB circuit during congenital heart surgery. In a small pilot study of 16 patients, the 8 patients randomized to receive 20 ppm iNO had shortened duration of mechanical ventilation and ICU stay compared to those receiving placebo (149). The potential beneficial effects of NO for ischemia reperfusion injury and inflammation due to CPB are discussed further below. There have been studies that examine the potential role of “NO precursors” in the acute and long-term management of pulmonary hypertension. Initial phase I and phase II trials using intravenous citrulline have demonstrated predictable pharmacokinetics and no safety issues so far with this approach (150).
In addition to the high cost of iNO, the agent has undesirable effects that should be carefully considered and where possible preemptively managed (151). The first of these is rebound pulmonary hypertension resulting from abrupt withdrawal of nitric oxide or rapid reductions in drug dosage. This is seen in both iNO responders and in nonresponders, and has been described after as little as a few hours of therapy. This phenomenon can be prevented with a careful weaning protocol that incorporates a single dose of the phosphodiesterase-5 inhibitor, sildenafil prior to discontinuation (152). High doses of iNO, prolonged use or inadvertent overdosing can all result in methemoglobinemia, which results from the binding of nitric oxide to hemoglobin (153). Therefore, methemoglobin levels should be routinely monitored, especially with prolonged therapy. Finally, nitrogen dioxide which can exacerbate or worsen lung injury, is a byproduct of nitric oxide administration, and its levels should be continuously monitored and maintained below 5 ppm (141).
Fenoldopam
Fenoldopam is a selective dopamine-1 receptor agonist with moderate affinity for α2-receptors. Despite its binding to α2-receptors, fenoldopam has no significant sedative effect. Fenoldopam administration produces dramatic vasodilation of the peripheral vasculature including renal, mesenteric, coronary, and skeletal muscle. The main indication for the use of fenoldopam is in the treatment of hypertensive emergencies and postoperative hypertension (154,155,156). The theoretical advantage of use of fenoldopam is that it maintains renal perfusion while decreasing blood pressure. This is especially important in patients with decreased renal function, since in these patients rapid drop in blood pressure may lead to decreased renal blood flow, GFR and even acute renal insufficiency. During prolonged infusion of fenoldopam there is development of tolerance with a half-life (predicted loss of 50% effectiveness of the drug) of 60 hours, without a prolonged pharmacodynamic effect or rebound hypertension upon discontinuation of fenoldopam. In a retrospective study of 25 postoperative cardiac neonates with inadequate urine output despite conventional diuretic therapy, fenoldopam increased urine output by 50% and had minimal effect on hemodynamics (157).
Nicardipine
Nicardipine is a dihydropyridine calcium channel antagonist that relaxes vascular smooth muscle by inhibiting calcium entry, resulting in a decrease in systolic, mean, and diastolic blood pressure and SVR
(158). Its negative inotropic effects are minimal, and compensatory tachycardia is less than for other direct vasodilators, that is, nitroprusside. It is effective for antihypertensive therapy in postoperative cardiac surgical patients with adequate LV function. Nicardipine has been used successfully for postcoarctectomy hypertension after repair via thoracotomy in children, with significant decreases in mean arterial pressure, and minimal increase in heart rate (159). Despite its lack of negative inotropic effect, this agent should not be used in patients with decreased systemic ventricular function, and should be used with caution in young infants.
(158). Its negative inotropic effects are minimal, and compensatory tachycardia is less than for other direct vasodilators, that is, nitroprusside. It is effective for antihypertensive therapy in postoperative cardiac surgical patients with adequate LV function. Nicardipine has been used successfully for postcoarctectomy hypertension after repair via thoracotomy in children, with significant decreases in mean arterial pressure, and minimal increase in heart rate (159). Despite its lack of negative inotropic effect, this agent should not be used in patients with decreased systemic ventricular function, and should be used with caution in young infants.
Beta Adrenergic Antagonists
Beta-blockers, like ACE inhibitors, are more beneficial in the management of chronic heart failure where they have been shown to improve the functional status in both pediatric and adult CHD (160,161). This effect is due to the modulation of the endogenous neurohumoral system. Several studies have demonstrated downregulation of beta-adrenoceptors in chronic heart failure as a result of elevated sympathetic tone (162,163). Therapy with beta-blockers such as propranolol, metoprolol, and carvedilol increase the number of myocardial beta-adrenoceptors and improve myocardial function in heart failure secondary to CHD (164,165). Responsiveness to catecholamines may be preserved in these patients during the perioperative period as a result of beta-blocker therapy. Besides their use in the management of chronic heart failure, these medications have several uses in the acute hemodynamic management of patients with CHD. By reducing the effects of increased sympathetic tone on the right ventricular infundibulum in TOF, beta-blockers are effective in the treatment of cyanotic spells (166). Also, a decrease in heart rate allows a longer diastolic filling time and improved preload. The use of esmolol, a short acting beta-1 selective antagonist, is well suited for the hemodynamic management of TOF in the perioperative setting (167). Esmolol in doses of 100 to 700 mcg/kg/min has also been successfully used to control postoperative hypertension after repair of aortic coarctation in children (125,168). Labetalol, a combined α– and β-adrenergic receptor blocker with 1:7 α:β blocking effect, is a frequent choice for intermittent IV dosing, during transition from continuous infusion β-blockade, to oral therapy. A labetalol dose of 0.1 to 0.2 mg/kg given every 1 to 4 hours is often effective.
Newer Cardiotonic and Vasoactive Agents
Currently, there exists limited scientific literature regarding the use of some new vasoactive drugs in patients with CHD, though a few of them have been well researched in other patient groups. A brief description of some of these newer agents is as follows:
Levosimendan
Levosimendan is an inodilator. Most other positive inotropes work through stimulation of adrenergic receptors and increase intracellular calcium that may be already elevated in the failing heart. Unlike these drugs, levosimendan works by causing conformational changes in the myofilaments making them more sensitive to intracellular calcium. The vasodilation produced is mediated by opening of potassium channels. Although drug is not approved for routine clinical use in the United States, there are extensive clinical studies involving large number of patients with end-stage cardiac failure demonstrating that levosimendan is both safe and effective in providing symptomatic relief. Levosimendan was also more effective in decreasing pulmonary artery wedge pressure and increasing cardiac output (169). Experience with the use of levosimendan in the perioperative period is limited. In a prospective randomized placebo-controlled trial in patients undergoing cardiac surgery, levosimendan given before separation from CPB enhanced cardiac performance, decreased SVR, increased myocardial oxygen consumption, and significantly decreased blood pressure, occasionally leading to hypotension (170). The hypotension was responsive to volume administration and did not require vasoconstrictors. In 15 children with acute heart failure, levosimendan improved ejection fraction in all while allowing a reduction in the dobutamine dose, in levosimendan doses of 6 to 12 mcg/kg load and 0.05 to 0.1 mcg/kg/min, with other hemodynamics unchanged (171). Despite exhibiting the expected hemodynamic effects, the drug has shown little or no effect on survival in adult patients with heart failure, and thus the drug has not been further developed for use in the United States. A recent review of 11 published studies of levosimendan use post pediatric cardiac surgery included three randomized controlled trials in 145 patients receiving levosimendan or milrinone after cardiac surgery (172). None of the three studies demonstrated a difference in clinical outcomes (length of mechanical ventilation and ICU stay) between the two agents. The largest study of 63 patients demonstrated lower inotrope scores and lactate levels on ICU admission with levosimendan. Complications due to inotropic regimen were not observed in either group. The other eight studies reviewed were case series of up to 293 patients; levosimendan was generally effective at improving LCOS, especially when no response to standard inotropes was observed. No adverse events attributable to levosimendan were noted.
Nesiritide (B-Natriuretic Peptide)
Nesiritide is a human recombinant form of BNP that is identical to and has actions that are similar to the endogenous BNP. Human BNP stimulates increases in intracellular cGMP in the vascular endothelial cells and smooth muscles. Elevated cGMP levels with nesiritide therapy lead to venodilation and arteriodilation. Nesiritide has natriuretic, diuretic, and vasodilatory properties. Currently, the primary use of nesiritide is in the treatment of acute decompensated heart failure. It produces dose-dependent reductions in the PCWP and systemic arterial pressure in patients with heart failure. In addition, vasodilation occurs without a change in heart rate and is associated with increases in stroke volume and cardiac output. In a randomized controlled trial involving 489 subjects, nesiritide when given as a bolus dose of 2 mcg/kg and followed by an infusion at 0.01 to 0.03 mcg/kg/min, was more effective in lowering the PCWP as compared to nitroglycerin (173). In another randomized controlled trial that included 103 patients with heart failure and systolic dysfunction, nesiritide was reported to decrease the PCWP by up to 39% as well as lower the right atrial pressure (RAP) and systemic vascular resistance, along with a significant improvement in the cardiac index (174). Colucci et al. (175) reported that nesiritide was effective as the standard drug therapy (dobutamine, milrinone, dopamine, or nitroglycerin) in treating heart failure. The renal hemodynamic effects of nesiritide (BNP) appear to be that of arteriolar vasoconstriction, which would likely augment GFR and filtration fraction in the setting of compromised renal perfusion. Additionally, BNP leads to an increase in both urinary sodium excretion as well as fractional excretion of sodium (FENA) (176). Mild diuretic effect of nesiritide therapy has been reported in a few clinical trials (176,177). In a limited pediatric experience of 17 patients after cardiac surgery, a loading dose of 1 mcg/kg was administered on bypass, and then an infusion of 0.1 then 0.2 mcg/kg/min was continued. There was a 7% decrease in mean arterial pressure, with no adverse effects (178). Use of this agent has been limited in the pediatric population, likely because multiple controlled adult trials have not demonstrated any survival benefit with this agent.
Mechanical Support
Patients requiring high-dose inotropic support to maintain marginal cardiac output must be considered for institution of mechanical support in the form of ECMO or VADs. High-dose support is
considered by many cardiac intensivists to be epinephrine, 0.1 to 0.2 mcg/kg/min (or its equivalent) for more than 2 to 4 hours. Mechanical support is ideally instituted proactively, rather than after a cardiac arrest or onset of multiorgan failure. See Chapter 25 for complete discussion.
considered by many cardiac intensivists to be epinephrine, 0.1 to 0.2 mcg/kg/min (or its equivalent) for more than 2 to 4 hours. Mechanical support is ideally instituted proactively, rather than after a cardiac arrest or onset of multiorgan failure. See Chapter 25 for complete discussion.